Hansen Solubility Parameters in Practice (HSPiP) e-Book Contents
(How to buy HSPiP)
Chapter 13 , That’s swell (HSP and Swelling)
If you look in the polymer data table you
can find the same polymer giving different values. This can be deeply troubling
to a first-time user and seems to undermine the whole premise of HSP.
Further thought reveals that some polymers must show different HSP under different
conditions. This is such an important principle that we give it a chapter to
itself.
For first-time users we provide an “Instant
Guide” set of values for common polymers. Until you’ve built up your own
experience, use these values for the polymers – but remember that they
are only there as an instant guide and that other values might apply for your
specific problem, as this chapter emphasises.
Let’s take for example PCTFE,
PolyChloroTriFluoroEthylene.
If you calculate a sphere using data from
solvents that swell it by >2% you get (though with so few good solvents, the
fit is somewhat arbitrary) [17.9, 2.9, 2.7], typical of a C-Cl polymer:
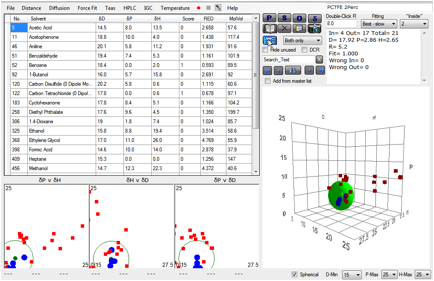
Figure 1‑1 A correlation with PCTFE swelling at 2% absorption
But if you do a plot with those solvents
that swell by >5% you also get a very different good fit, typical of C-F
polymers [15.6, 4.9, 7.5].
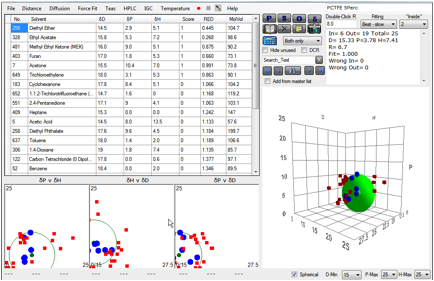
Figure 1‑2 Same polymer, different correlation at 5% absorption
What’s happening is that at low levels of
solvent absorption, the solvents associate themselves with the –Cl rich
areas of the polymer. As you go to greater swelling, the solvents have to
associate with the predominant C-F regions.
This must be a general principle. If you
test a polymer which contains a small portion of –OH functionality then
at low levels of swelling, alcohols will be very happy to be associated with
these regions, so the solvent sphere is biased towards the alcohol region. But
when you start swelling/dissolving the whole of the polymer, the alcohols are
very poor solvents, so the sphere shifts towards a lower δH and δP region.
Similarly, if a polymer contains
crystalline and non-crystalline regions, then swelling data at low levels of
solvent will reflect the non-crystalline region and therefore a bias towards
whatever functionalities preferentially reside in that region.
So we can now flip the problem of having
different solvent spheres into a distinct advantage. If you find conflicts in
the data, these may well be providing you with fundamental insights into the
internal structure of the polymer. It’s not obvious that PCTFE should have
chlorine-rich and fluorine-rich regions, but the HSP data seem to suggest that
that is the case.
The same principles can be applied to the
latest nano-scale issues. It is becoming common practice to e-beam write
nanostructures for integrated circuits, photonic crystals and nanobiology. When
“negative” resists are used (i.e. those that become less soluble on exposure)
there is a problem of development. You want a solvent that quickly whisks away
the un-crosslinked resin. But such a solvent can readily enter the cross-linked
polymer and cause it to swell. If you write 10nm features, then it only needs
swelling of 5nm across both sides of the feature and the swollen polymers touch
across the divide and degrade the quality of the image. One proposal to fix
this is to use solvents just at the edge of the HSP sphere – they will
still dissolve the un-crosslinked resin, but will be unlikely to enter the
crosslinked system. We are grateful to Dr Deirdre Olynick and her team at
Lawrence Berkeley National Laboratory for allowing us to reproduce data from
their paper that explores these issues in a profound way: Deirdre L. Olynick,
Paul D. Ashby, Mark D. Lewis, Timothy Jen, Haoren Lu, J. Alexander Liddle,
Weilun Chao, The Link Between Nanoscale
Feature Development in a Negative Resist and the Hansen Solubility Sphere, Journal
of Polymer Science: Part B: Polymer Physics, Vol. 47, 2091–2105 (2009).
The team first established the HSP sphere
for the calixarene resist of interest.
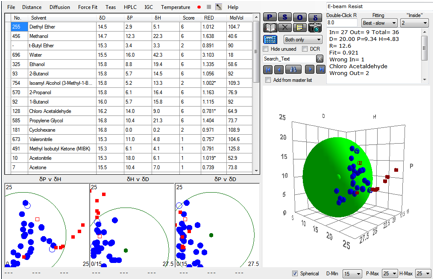
Figure 1‑3 Sphere for Calixarene e-beam resist
It is interesting to note that they used a
sophisticated Sphere algorithm (fully described in their paper) which included
some heuristics that could eliminate false fits. Happily, the values of our
straightforward algorithm match theirs. They were then able to show that
solvents closer to the centre of the sphere were better at creating high
contrast images, whilst those near the edge were better at avoiding the
problems caused by swelling. A rational compromise can then be reached on this
basis. Importantly, other solvents and/or solvent blends can then easily be
devised on rational principles to improve the process even further. The paper
contains much more of interest and readers are recommended to explore their
paper in detail.
Of course kinetics must be part of the
optimisation process and it is likely that issues discussed in the Diffusion
chapter will also play a part in understanding. But by establishing the basic
thermodynamics of the system, further optimization can be a more rational
process.
E-Book contents | HSP User's Forum


