Hansen Solubility Parameters in Practice (HSPiP) e-Book Contents
(How to buy HSPiP)
Chapter 27, Liquid extraction – a work in progress
There are two ways to look at the problem.
The first has the merits of simplicity, clarity and fitting in with the general
approach of HSPiP. The second is theoretically interesting but shows some
limitations to the first approach which we are doing our best to understand.
The
simple approach
Let’s assume that you have a bulk material
containing your chemical of interest. All simple processing (e.g. filtration)
has taken place with the bulk and any obvious way to extract the good stuff via
a simple process (e.g. crystallisation from a simple solvent) has been
excluded.
So you have two problems. The first is to
dissolve the good stuff. The second is to not dissolve the unwanted stuff.
From the HSP of the good stuff you will
quickly identify a set of good solvents from their close HSP match – the
Solvent Optimizer will do that nicely. The real difficulty is with the bulk.
Here the recommended way forward is to do a classic 20-test tube Sphere
experiment to get an approximate centre and radius for the bulk. Then you need
to select an extraction solvent that is sufficiently near the edge of the
Sphere yet sufficiently close to the good stuff to be effective.
One subtle variation of this goes back to
the old “problem” of Sphere radii. Suppose you want to extract from a polymer.
If you do a Sphere test based on solubility you will get a small radius. If you
do the test based on swellability you will get a large radius. [In practice you
would just do one test but score “soluble” as 1 and “swellable” as 2 and
calculate the Sphere with a 1 then a 2 as the criterion]. You can now choose your
extraction solvent as one that is both reasonably good for your target compound
and also in the swellability portion of the Sphere so it has a chance to get
inside the polymeric mass to do its extraction.
If you find that there is insufficient
distance between the bulk and the good stuff then it’s highly likely that
liquid extraction isn’t a great method for this particular mix.
Of course you might be lucky. If you are
doing water/solvent extraction it might be that the bulk material is
specifically biased towards the water, in which case your choice of solvent
will be based on HSP distance, water immiscibility and then
cost/safety/environment considerations.
The point of this section is that although
HSP may not provide all the answers they do provide a rapid means of assessing
probabilities of success or failure.
The
theoretical approch
The calculation of liquid/liquid partition
ratios would, from simple theory, depend on MVol and HSP Distance2,
i.e. the chi parameter. So what’s stopping us from providing a liquid
extraction modeller to HSPiP?
There are two key problems here. The first
is that the water can sometimes contain some solvent and the solvent can
contain some water. The classic example is octanol/water where the water
contains essentially no octanol but the octanol contains 20% (molar) water. The
second is that water itself is so odd. As discussed elsewhere in this eBook,
the solubility of many molecules in water is influenced less by HSP distance
and more by the “hydrophobic effect” which is mostly a size-related issue, so
that larger molecules are generally much less soluble in water unless they
contain plenty of alcohol, amine or acid functionality or are salts.
Because octanol/water is the most familiar
partition coefficient, it’s worth plotting the (non) correlation between LogKD
and HSP distance from octanol:
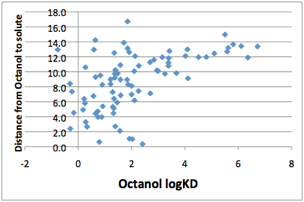
Figure 1‑1 No correlation between Octanol distance and Octanol/Water partition
As is well-known, the single best predictor
for LogKD is molar volume, and here is the proof:
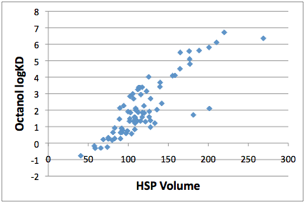
Figure 1‑2 A good (and well-known) correlation of Octanol/Water with MVol
So much is well-known. What is less
well-known is that there are some intriguing correlations of water/solvent
partition coefficients with HSP. Here are the data for hexane:
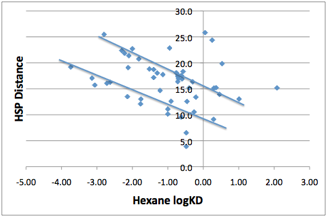
Figure 1‑3 Two correlations seem to be present in the Hexane/Water partition
coefficient
The two correlation lines in there need
urgent explanation, as do those very scattered points that are clearly outside
any correlation.
The upper line is for alcohols and amides:
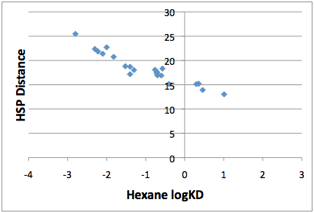
Figure 1‑4 The upper line is a good plot for alcohols. The lower line (not
shown) is from carboxylic acids, esters and ketones
The lower line comes from carboxylic acids,
esters and ketones. The scatter comes from amines (which are highly chaotic)
and then (mostly) from compounds such as vanillin or hydroquinone with hydroxyl
groups plus other functionality. We can perhaps excuse the amines as their
state of protonation will make a large difference in ratios, and small amounts
of spare acid or base in the system can make large changes to the ratio. This
leaves us with the hydroxy-containing molecules and the problem of alcohols and
amides.
The Ruelle method for calculating the
hydrophobic effect seems to offer a good explanation for the
hydroxyl/alcohol/amide exceptions. Their (in)compatibility with water is
modified by their ability to hydrogen bond with the water molecules and
therefore reduce the hydrophobic effect. They are therefore more soluble than
their HSP distance would suggest. The problem arises in multi-functional
hydroxyl molecules. If, for example, they have strong intra-molecular hydrogen
bonds, they will appear more like non-hydroxy molecules to the water so will be
less soluble than a similar molecule (e.g. an m-hydroxy instead of an
o-hydroxy) which has no intra-molecular hydrogen bonds.
Solvents such as carbon tetrachloride and
benzene show similar tendencies to hexane, so predicting liquid-liquid
extraction should be possible if HSP distance and a Ruelle correction term are
included in an overall formula. But chloroform and diethyl ether both show a
different set of non-conformities. We are currently investigating possible
root-causes for the problems with these solvents. A potential problem is
induced dipoles as witnesses by variable dipole moments depending on the local
environment (see pp. 16-17 in the handbook).
So of the 6 solvents for which we have
adequate test data:
·
3 (hexane, carbon tetrachloride
and benzene) look as though there’s a possible HSP-based formula if Ruelle
hydrophobic corrections can be included.
·
1 (octanol) is mostly fit with
a pure hydrophobic calculation (MVol)
·
2 (chloroform, ether) are not
yet explained.
This is not a good starting point for those
who want a generalised model for liquid-liquid extraction with other solvents!
Almost certainly the deviations from HSP
predictions would be a lot less if neither liquid is water. At the time of
writing we don’t have a dataset for such systems with which to test the model.
As this is such an important area, the
HSPiP development team will continue to explore options to create a robust
modeller to add to the software. If we do, then this chapter will be
significantly revised!
E-Book contents | HSP User's Forum


