Hansen Solubility Parameters in Practice (HSPiP) e-Book Contents
(How to buy HSPiP)
Chapter 7, Coming together (Polymer Compatibility)
How do you get immiscible polymers working
together? A good solvent for one will naturally be a bad solvent for the other
so there is a genuine difficulty in finding a way of bringing them together.
For polymers which aren’t too far apart, it’s still
fairly easy to make a good guess and get a result. But what happens if they are
seriously immiscible?
A good example is when you want to combine
the properties of a silicone with those of another polymer. In order for the
final system to be stable, there must be some reactive groups on both so that
mutual cross-linking ensures that the phases can’t separate. But because it
needs only a relatively small amount of cross linking we can assume that the
polymer HSP are the standard, unmodified ones even though the real polymers
would have a few percent of some reactive group such
as an alcohol, an amine or a methacrylate.
A typical HSP test for a real-world
silicone shows values around [17.1, 2.2, 3.1, 5.7]
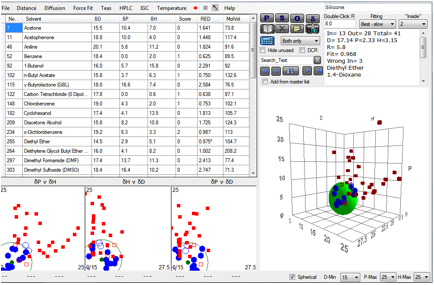
The challenge is to compatibilise
it with an epoxy [17.4, 10.5, 9, 7.9]
The distance between these two is 9.6 so
their centres are each outside each other’s sphere. These are genuinely
incompatible.
When the two polymers are entered into the
Polymer table and selected, then the Solvents button clicked, the following
appears:
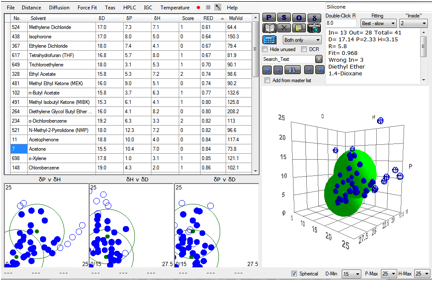
You immediately find some helpful solvent
suggestions. Something volatile such as MEK or THF will be ideal for generally
allowing the polymers to mix, but you also tend to need some “tail” solvents to
hang around whilst the reaction is finishing so something like Cyclohexanone would seem a good idea.
Readers might be a bit disappointed at how
easy this seems. But the authors’ experience is that without the convenience of
HSP thinking (and HSPiP) this sort of problem has
involved many months of avoidable work by major corporations.
Self organisation
You can use the same sort of ideas to do
some clever self-organising coatings. Suppose you want an acrylate polymer as
the top surface of a dual-layer coating with an epoxy at the bottom. Of course
you could make this a two-pass coating. But in some applications a one-pass coating, if
feasible, would save lots of time and money. So what we
would like is a spontaneous separation of the components when the solvent
evaporates. But for this to happen the solvent must be rather poor for both of
them. Hence the RED number for each polymer with the solvent should be in the
0.8-1.0 range. The polymer with the lower surface
tension is expected to be at the air surface then becoming the topcoat if
motion within the film allows this. Its further accumulation at the air surface
to achieve a significant thickness is then enhanced by a reduced affinity for
the other polymer, which then forms a primer. Clearly other surface
active components in the coating can interfere with this.
The Polymer form provides an Acrylate
[20.7, 4.1, 10.7, 11.5] and an Epoxy [18.5, 9, 8, 9.8]. If you select Friendly
Solvents in the Solvent form then when you select the Acrylate and click
Solvents you find that the RED number for Xylene is 0.89. Selecting the Epoxy
and clicking Solvents gives a RED of 0.97 for Xylene.
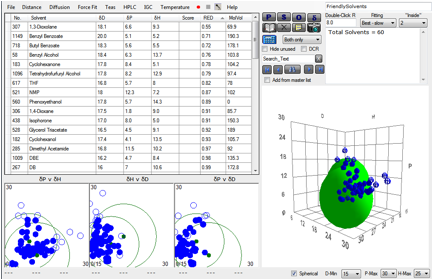
This example is a simplified version of a
real dual-layer coating. As the Xylene evaporates, the mutual incompatibility
of the two polymers becomes evident and phase separation begins. The lower
surface energy of the acrylate polymer brings it to the surface. The phase
separation continues till you have an almost perfect dual-layer. The “almost”
is important. In order to preserve adhesion, there must be some intermingling
of the polymer chains at the interface. The kinetics of the system ensure that the phases don’t separate totally.
Again, this seems easy in retrospect, but
if you tried to do this without the aid of HSP thinking it would take a very
long time to get even the basic functionality working correctly.
Non-solvents
coming together
One of the striking and unexpected
predictions from HSP is that mixtures of non-solvents are perfectly capable of
being excellent solvents. Hansen showed this back in 1967 when working on the
polymer series for which we’ve provided up-to-date correlation data in files
Polymer88xx. By choosing Polymer88E you find its HSP are [19.3
,6.0, 10.4, 10.5]. If you check the data table you find that Diethyl
ether [14.5, 2.9, 5.1] and Propylene carbonate [20.0, 18.0, 4.1] are both
non-solvents. But a 50:50 mixture [17.3, 10.5, 4.6] is inside E’s sphere and is
calculated to be, and was shown in practice by Hansen to be, a solvent. There
are numerous examples of such mixtures of non-solvents being solvents and it is
important for formulators to think outside the box (or, rather, outside the
sphere) and start to get different solvency characteristics by mixing solvents
they would otherwise have totally ignored. It’s worth saying once more that the
Hildebrand solubility parameter simply cannot do this sort of thing because
there is no coherent way of dealing with the issue that very different solvents
can have very similar Hildebrand parameters.
E-Book contents | HSP User's Forum


