Organo-fluorine compounds, PFOA, PFOS contamination is serious in Okinawa and Yokosuka in Japan.
Can we say that water purified with activated carbon is safe to drink?
It was originally used as a fire extinguisher (foam extinguisher) in fighter planes. It is a surfactant suitable for making permanent foam in small amounts.
When it leaks into the environment, can it be adsorbed by activated carbon? How would you answer the question?
For example, formaldehyde and acetone, which have become a problem in sick house syndrome, cannot be adsorbed by activated carbon.
In the world of chemical engineering, we would learn that hydrophilic substances that dissolve in water (acids and bases) should be absorbed in absorption columns, while hydrophobic substances should be absorbed in activated carbon adsorption columns.
The fluorine part is hydrophobic and the carboxylic acid part is hydrophilic, and if it is a Li, Na, or K salt, it would be very hydrophilic.
If you are doing MI/AI/ML, you should be able to give your own answer with reasons in 30 minutes.
As training data,
Use a 1986 paper entitled Estimation of Activated Carbon Equilibrium Adsorption by Adsorption Index (AI).
Let’s type it up and do it ourselves!
I’d like to say.
but I don’t think anyone will do it first.
Let’s do it by copying and pasting the following data.
With this data, you can get the information you need in 5 minutes if you have HSPiP, or 15 minutes if you do RDKit or molecular orbital calculations.
If you are a chemistry major and can read molecular names and structures to some extent, you can easily see that compounds with aromatics, chlorine, and bromine have large logK (adsorption constant).
For example, you would create an identifier in RDKit using the SMILES structural formula, and then repeat drawing the graph with log K.
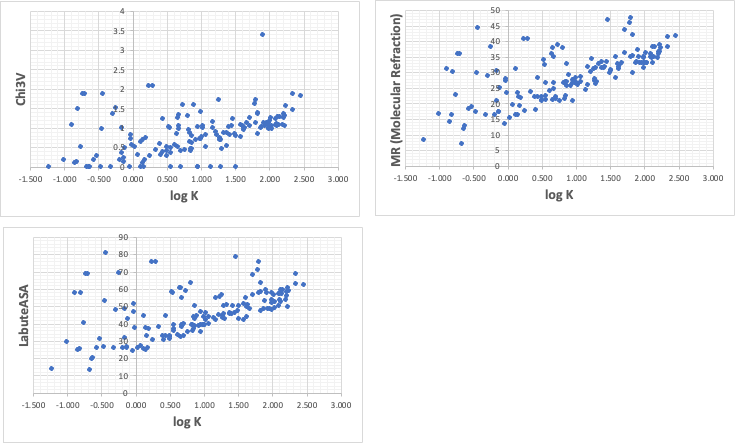
The three items above that seemed to be relevant were Chi3V, LabuteASA, and MR (Molecular Refraction).
And,
PFOA( 2.60, 124.7, 43.37)
PFOS( 3.92, 145.7, 52.2)
Let’s put these values in the above graph and estimate how much logK is.
If you have HSPiP, try to calculate tot HSP/MVol.
tot HSP=sqrt(δD2 + δP2 + δH2)
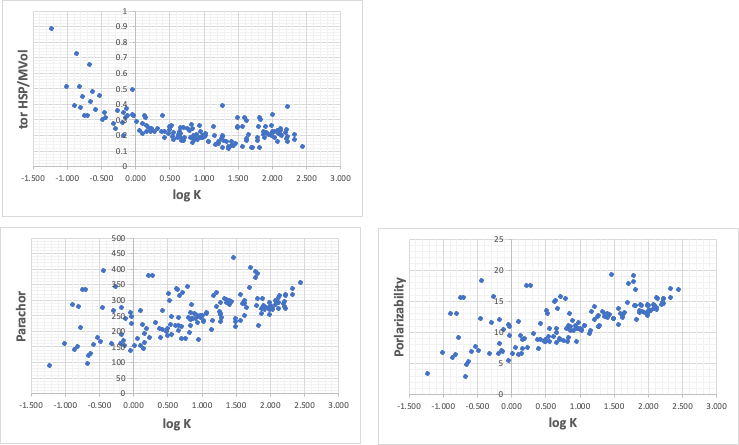
Parachor and polarization rates for calculating surface tension are also highly correlated.
PFOA( 0.062, 496.7, 19.18)
PFOS( 0.06, 608.86, 20.13)
Put these values into the above graph to estimate how much logK is.
We often use MO as an identifier, as it can be used to calculate any molecule for now.
However, when I draw a graph like this, there is not much correlation.
Even if there is no correlation in each of the 150 or so molecules in this calculation, the neural network will find the equation by multivariate analysis, although the contents are a black box.
However, in many cases, it collapses when the data increases.
If you don’t find a single correlation as much as possible and get into the habit of thinking why it has a correlation, you will wither as a researcher.
If you have a pirika analysis tool such as MIRAI or GROVE, create a QSAR equation.
Let them automatically select the important items and create the equation.
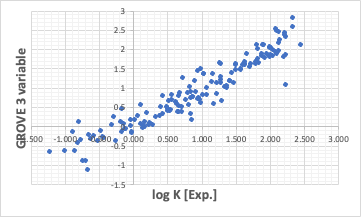
If we choose three variables and create an equation, it will look like this.
After that, quantitative values can be obtained by putting the values of PFOA and PFOS into the equation.
It is similar to Random Forest’s method, but if you create a number of equations and all of them say that the log K of PFOA, PFOS is high, you can finally drink water purified with activated carbon with peace of mind.
However, this is a calculation for acid-type molecules; if they are in Li, Na, or K salt, their solubility in water will be much higher, so it will not work this way.
In addition, the concentrations of PFOA and PFOS in the environment are very thin.
Moreover, the world is full of hydrophobic molecules that are easily adsorbed competitively.
Try it with regular soaps with or activated charcoal in the fridge.
If the surfactant is adsorbed, it will not foam.
