2024.9.04
Chemistry at pirika.com > Chemstry > Chemical engineering > Reprint: gas-liquid equilibrium estimation by ASOG. > Chapter 1: Basic equations of solution theory
When a given liquid is present, the molecules in the liquid are in motion and collide with each other. Robert Brown (1827: Brownian motion) observed this in the movement of pollen. Unlike gases, liquids are very crowded, and if they move even slightly, they collide with other molecules. The collisions cause the molecules that have been ejected from the liquid phase to fly out into the gas phase. Pressure is observed when such gases collide with walls. This is the vapour pressure. As the temperature of the liquid is increased, the movement of the molecules becomes more intense and more molecules are ejected into the gas phase. Some molecules also return from the gas phase to the liquid phase.
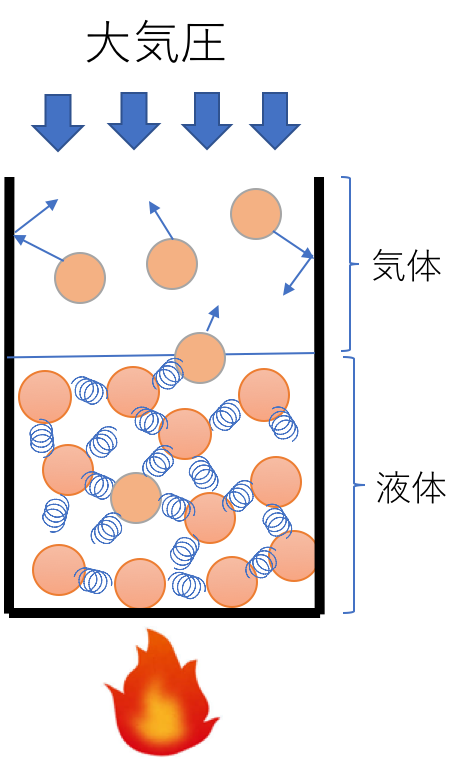
The point at which the vapour pressure equals atmospheric pressure is defined as the standard boiling point. The vapour pressure diagram plots the relationship between this temperature and vapour pressure. As pressure increases exponentially, the vapour pressure axis often takes the logarithm (log). As an example, the vapour pressure diagram of ethanol is shown below.
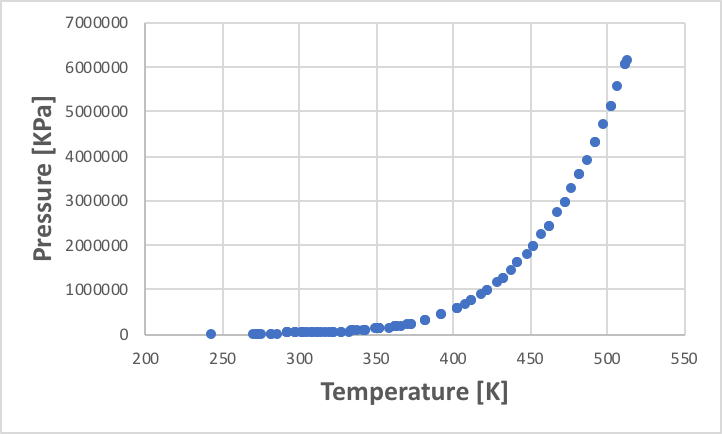
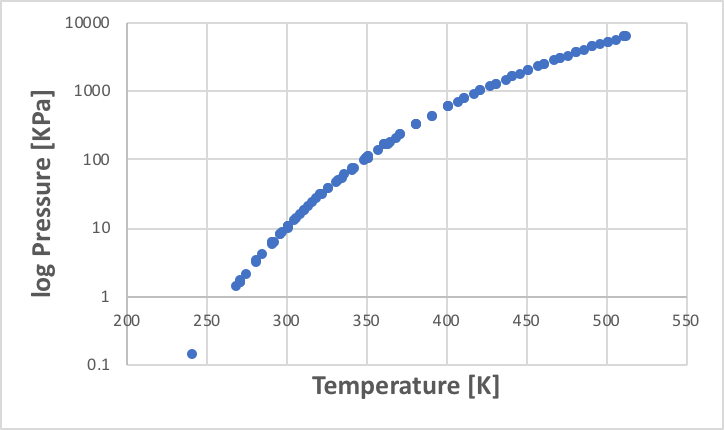
The vapour pressure of a given liquid is determined to one degree once the temperature is determined. However, this is not so easy when it comes to mixtures. For example, water boils at 100 °C and ethanol boils at 78.2 °C. Then at what temperature does a 50% molar solution of ethanol boil?
(100+78.2)/2 at 89.1°C?
In an ideal solution, Raoult’s law holds that ‘the vapour pressure of any component is equal to the vapour pressure of that component when it exists alone multiplied by the mole fraction of the solution’.
Total pressure P=P1*x1+P2*x2 Raoult’s law.
(In a two-component system,x2=1-x1.) (1-1)
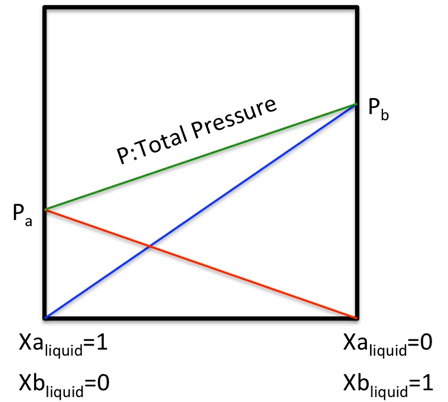
This means that whatever concentration ratio is used to mix the two components, the ratio of the liquid phase to the gas phase of the two components will be the same, as shown in the diagram below.
So, for the question ‘At what temperature does a 50% molar aqueous solution of ethanol boil?’,
Atmospheric pressure = P ethanol*0.5 + P water*0.5
So we can find the temperature at which
As it is difficult to read off the pressure, P ethanol and P water at a certain temperature T from a vapour pressure diagram, a representation of this vapour pressure curve as a function of temperature is used. The most prominent correlation equation is the Antoine vapour pressure equation.
Water: logP(mmHg)=7.4155-1348.12/((T[℃]+197.68) ) (1-2)
Ethanol: logP(mmHg)=7.5424-1286.85/((T[℃]+197.20) ) (1-3)
With these equations, the pressure, Pethanol and Pwater at any temperature T (°C) can be obtained.
Calculating the vapour pressure at the average value of 89.1 °C mentioned earlier gives water (518.34 mmHg) and ethanol (1115.93 mmHg), which gives a vapour pressure of 817.14 mmHg for the mixture calculated using Raoult’s law.
It is slightly larger than atmospheric pressure (760 mmHg).
At 85°C, it is too small at 701.59 mmHg.
At 88°C, a little too big at 784.72 mmHg.
At 87.135°C, it is 759.97 mmHg, almost 760 mmHg.
This method of calculating with an appropriate temperature and then increasing or decreasing the temperature to find the optimum temperature by looking at the results is called a convergence calculation. It is difficult to do manually, but an answer can be easily obtained using an Excel solver.
The important point is that near the boiling point, a change in temperature of (89.1-87.135) = 1.965°C will change the vapour pressure by 817.14-760 = 57.14 mmHg.
This corresponds to approximately 30 mmHg per 1°C. This should be kept in mind.
Now, if a 50% molar solution of ethanol boils at 87.135°C, what would the composition of the vapour phase be?
The assumption used here is that ‘at pressures as low as atmospheric pressure, gases behave as ideal gases’.
Next section: 1.2 To the ideal gas
Copyright pirika.com since 1999-
Mail: yamahiroXpirika.com (Replace X with @.)
The subject line of the email should start with [pirika].
