2024.9.04
Chemistry at pirika.com > Chemistry > Chemical engineering > Reprint: gas-liquid equilibrium estimation by ASOG. > Chapter 1: Basic equations of solution theory > Chapter 2: ASOG method. > 2.3 Actual ASOG method calculation
2.3.3 Gas-phase association system
Small molecule carboxylic acids are known to evaporate in dimers. Carboxylic acids are hydrogen-bonded compounds and originally out of ideal solution. If carboxylic acids form three-dimensional hydrogen-bonded networks, like water and alcohols, they do not need special treatment.
The peculiarity of carboxylic acids is that they form dimers and their hydrogen bonds are so strong that they remain dimers in the gas phase even after evaporation: the polar parts of COOH cancel each other and behave as if they were one non-polar molecule. What happens when this happens?
According to the Boyle-Charles law for ideal gases, PV = nRT (n: number of moles, R: molar gas constant). For simplicity, let us assume 100% association in the gas phase.
Once the temperature, pressure and volume are determined, the number of moles of molecules in the gas phase is constant: when two molecules behave as if they were a single molecule, the number of moles is apparently the same as that of unaggregated molecules. However, when quantified by gas chromatography or other means, twice as many moles are present in the gas phase. Therefore, an equilibrium constant is needed to determine how much is aggregated in the vapour phase.
The equilibrium constant is a constant that indicates how much of the equilibrium reaction, 2A ⇆ A2, is present.
Measured values of the gas-phase association equilibrium constants for typical carboxylic acid compounds are as follows.
formic acid: log K= -10.743 + 3083.0/T (323 K ~ 423 K) (2-9)
acetic acid: log K =-10.931 + 3347.0/T (323 K ~ 423 K) (2-10)
propionic acid: log K= -10.834 + 3316.0/T (323 K ~423 K) (2-11)
What do we actually do with this constant? In the same way as in the vapor-liquid equilibrium treated in the previous section 2.2.3 Heterogeneous systems, we will consider the vapor-liquid equilibrium and use the ‘tendency to escape from the phase’ fugacity to form an equation.
Essentially, the following three equilibrium constants must be obtained to find the vapour-liquid equilibrium of a two-component system.
2A ⇆ A2
2B ⇆ B2
A+ B⇆ AB
The original programme shows that this has been addressed. And the equilibrium constants are to be input manually. However, equilibrium constants other than those for formic acid, acetic acid and propionic acid are not shown. In addition, even if only the equilibrium constants for carboxylic acids are entered, the results in the book have large errors compared to those for other groups. Because of this, the programme in the book does not support vapour phase association.
The list of examples involving carboxylic acids is as follows.
(21)(22)(23)(24)(48)(72)(73)(102)(103)(104)(114)
As a test, let us calculate (21) Acetic Acid/Octane.
It can be seen that the mole fractions diverge significantly around the middle.
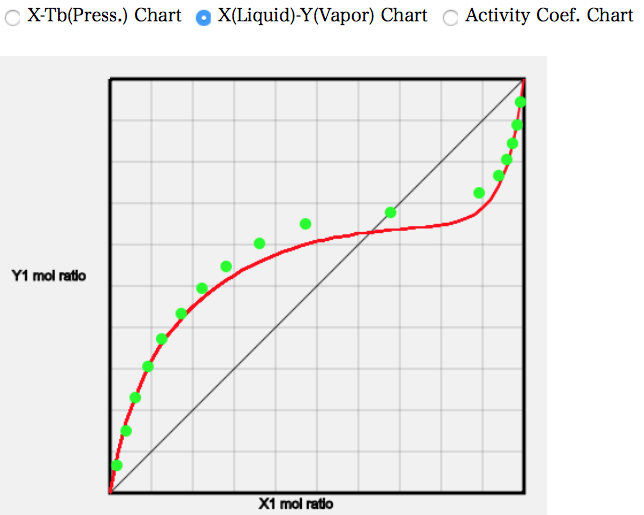
Thus, basically, solvents with carboxyl groups have a large error margin.
This difference is the only reason why so much aggregated acetic acid is in the gas phase. Conversely, once these data have been collected, the gas-phase association constant can be determined.
The larger the molecule, the more it cleaves in the gas phase, so it becomes an ordinary polar molecule.
Copyright pirika.com since 1999-
Mail: yamahiroXpirika.com (Replace X with @.)
The subject line of the email should start with [pirika].
