2024.9.04
Chemistry at pirika.com > Chemistry > Chemical engineering > Reprint: gas-liquid equilibrium estimation by ASOG. > Chapter 1: Basic equations of solution theory > Chapter 2: ASOG method. > 2.3 Actual ASOG method calculation
2.3.4 Evaluation of the azeotrope by the ASOG method.
In the book, the azeotropes of alcohols and hydrocarbon compounds determined by the ASOG method are compared with the measured azeotropes. Many of the same solvent pairs are described, with constant pressure but different pressures, constant temperature but different temperatures, etc. So the real solvent pairs are very limited.
As alcohols, compounds containing isomers from C1 to C4 are used. As hydrocarbons, chain, cyclic and aromatic hydrocarbons from C1-C8 are used.
As the composition and temperature of these azeotropes are not given in the books, we will use values from a separately constructed database of azeotropes at ambient pressure.
As an example, let’s look at the calculation of (12)Ethanol/Toluene. The programme searches the database for a given solvent pair. The order of precedence is the database of measured vapour-liquid equilibria, the database of Wilson parameters, and the database of azeotropes, in that order. Thus, if the solvent pair is found in the database of measured gas-liquid equilibria, a number of green (light blue) data points of the experimental values are displayed.
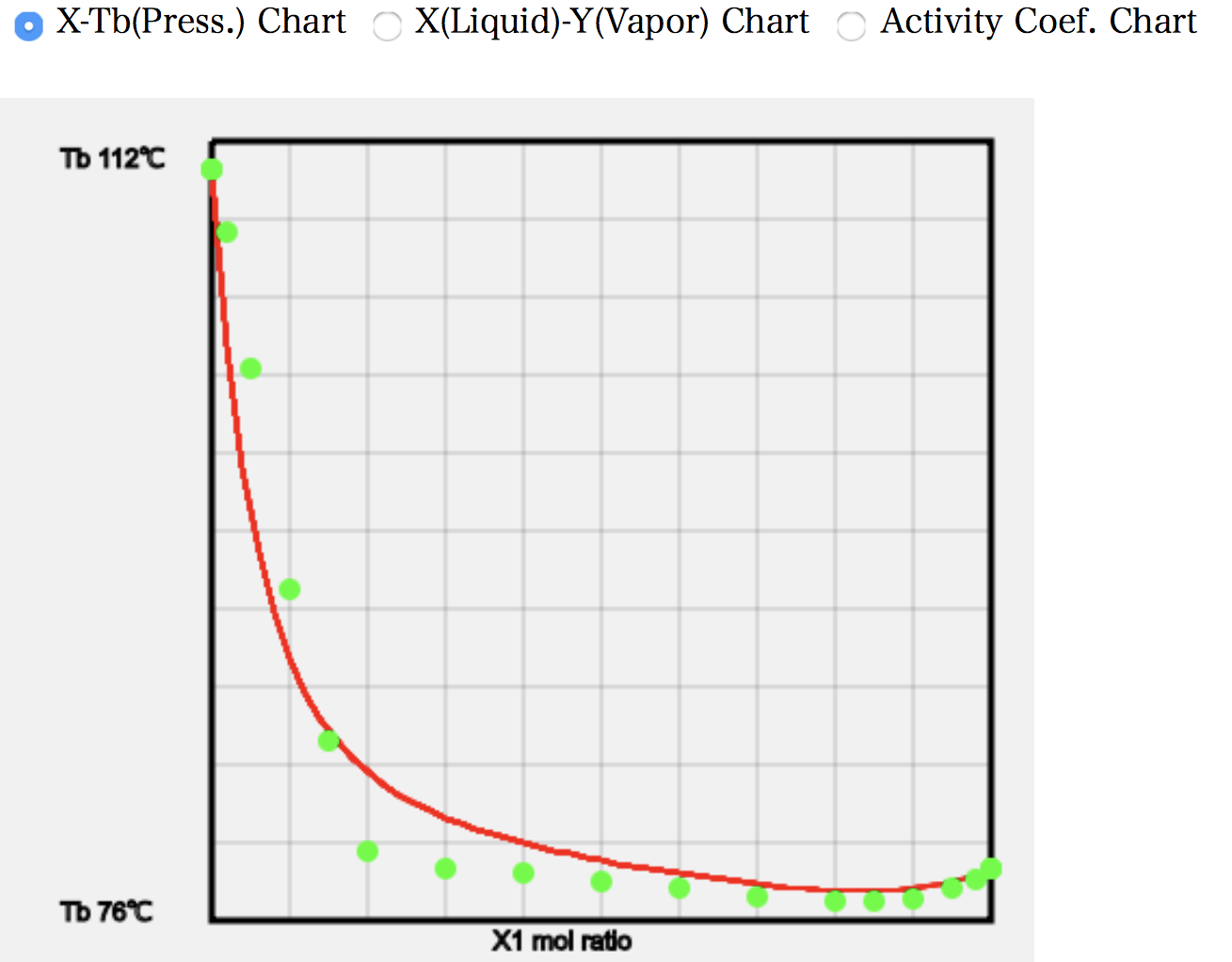
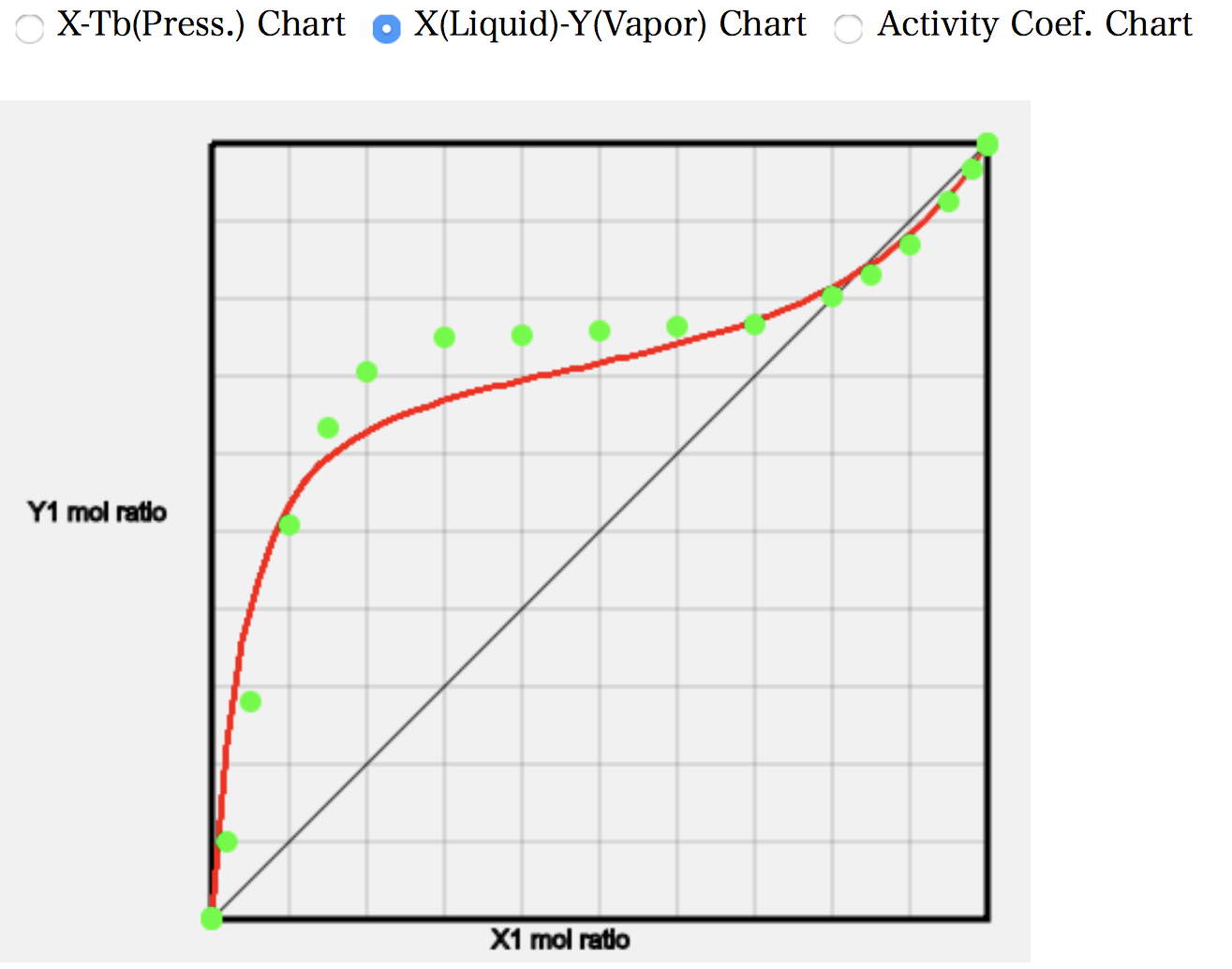
The point at which the composition of the gas and liquid phases coincides is well matched, with a DB value of 0.818 but an ASOG estimate of 0.832.
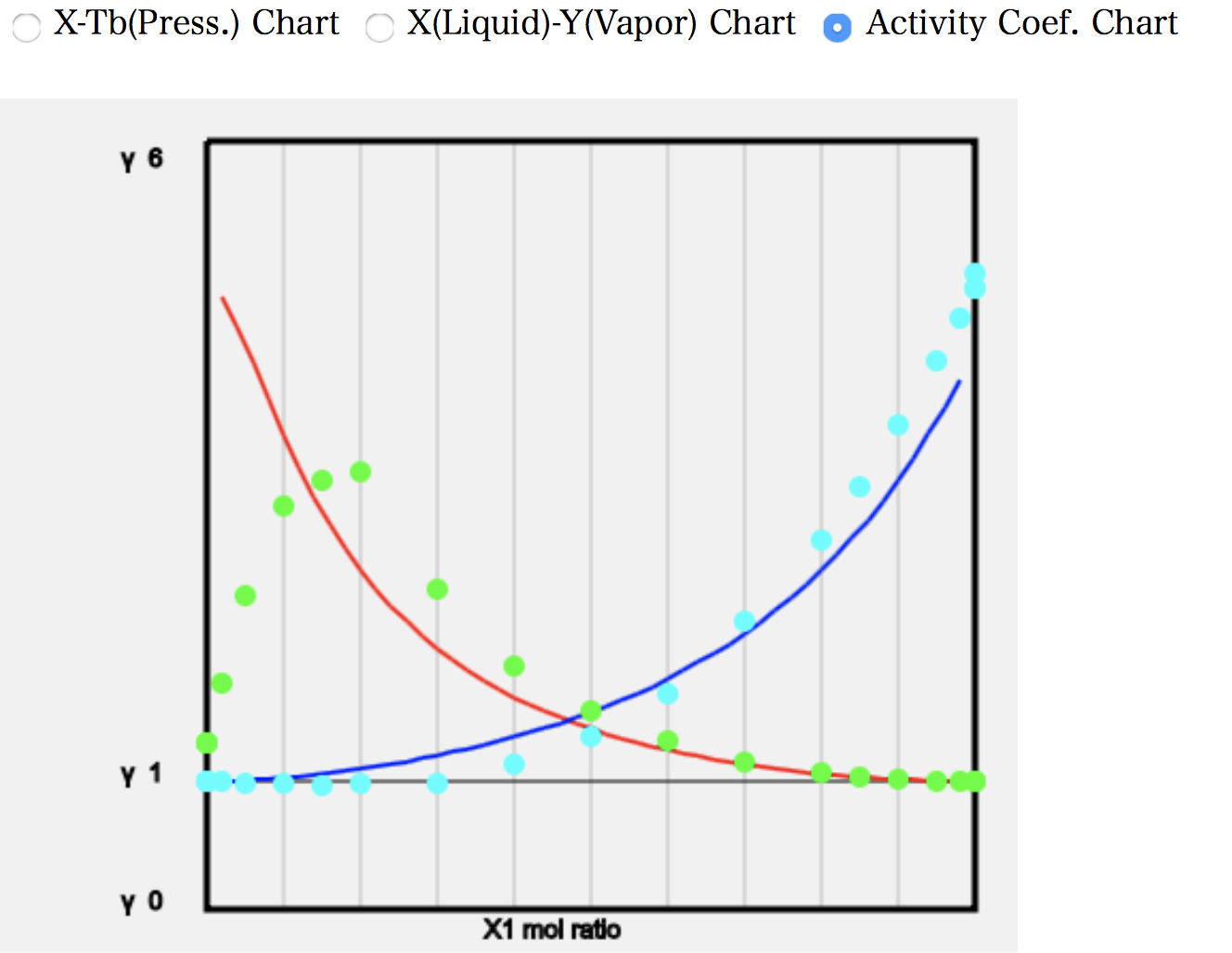
All results are plotted as follows.
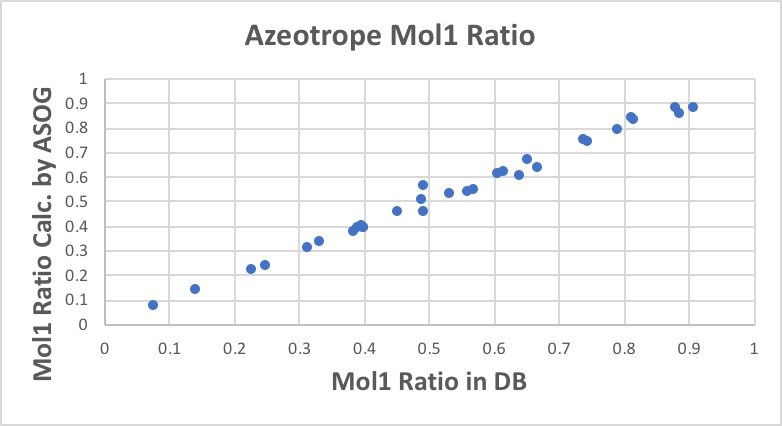
With regard to the composition of the azeotropes, the results are very good, except for (7) heptane/1-propanol.
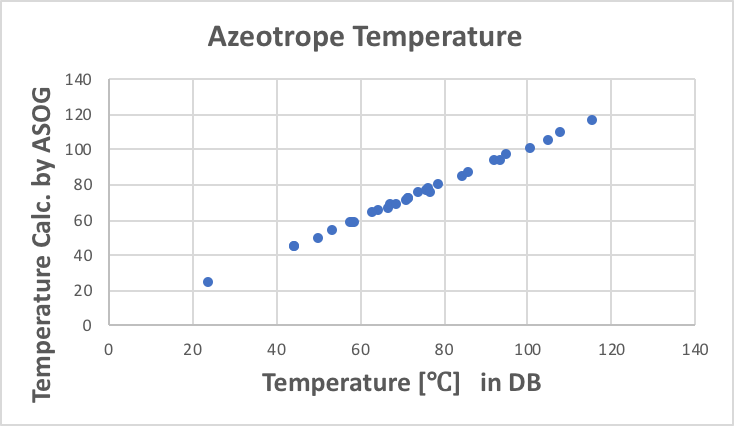
With regard to the azeotrope temperature, the results are almost problem-free.
Let me show you how this can be useful.
For example, look at the calculation of (25) 2-Propanol/iso-Octane.
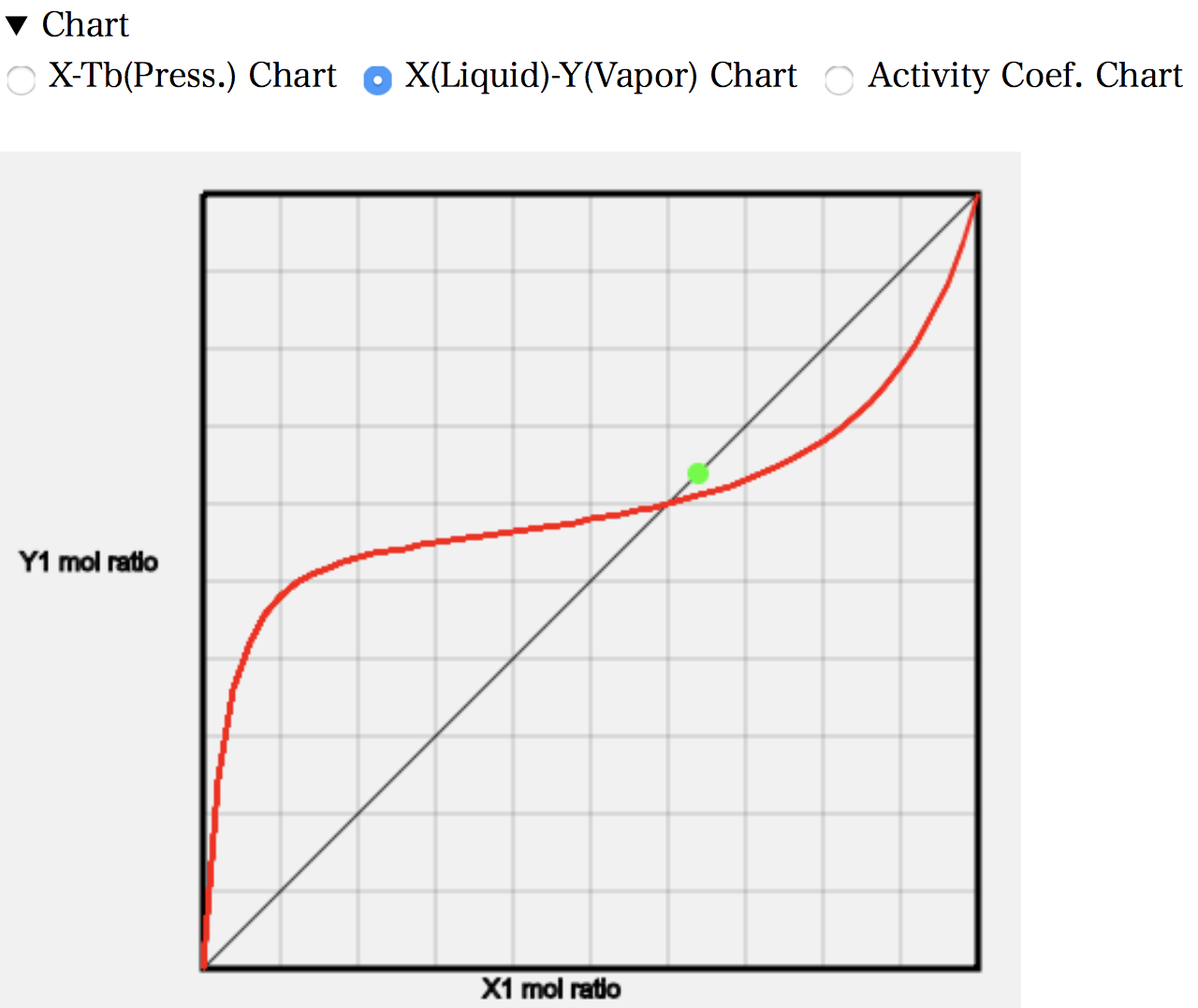
For this system, only azeotropic data are available. So, although there is only one actual measurement point, it can be seen that the measured and estimated values are in good agreement.
Iso-Octane is the main component of petrol. Petrol is introduced into the engine compartment and vaporised. It is then ignited by the plug and burns, pushing the piston under pressure. How the vaporisation takes place is important.
Recently, bio-ethanol has been added to gasoline in Brazil and the USA due to environmental concerns. What is the vapour-liquid equilibrium between ethanol and iso-octane? It is very important to predict this from computers alone.
Gasoline is a mixture of about 300 different compounds, including trace elements. Aromatic compounds are sometimes added as high-performance petrol. 2-Propanol is sometimes added as a draining agent in petrol tanks; it would be impossible to measure all two-component systems. And if it becomes a multi-component system, it will be impossible to do anything about it.
It is important to obtain activity coefficients using the ASOG method, which is as accurate as possible and can be extended to multiple components.
I have added iso-octane solvent pairs to the examples. For some pairs, there is no measured data at all, but you can try and calculate them.
(32) Methanol/iso-Octane
(33) Isobutanol /iso-Octane
(34) 1-Propanol/iso-Octane
(35) Ethanol/iso-Octane
In Japan, MTBE and ETBE are being used instead of ethanol because ethanol tends to absorb moisture and rust. It is not known whether these will be the ones from the point of view of toxicity issues and production costs, but it is worth checking the vapour-liquid equilibrium of these compounds.
(36) Methyl tert-butyl ether (MTEB)/iso-Octane
(37) Ethyl tert-butyl ether (ETBE)/iso-Octane
(If you have data on azeotropy and vapour-liquid equilibrium for MTEB and ETBE, we would be glad to hear from you. We would like to reflect this in the revision of this publication).
Other advantages include the fact that when vapour cleaning is used to clean electronic substrates, an azeotropic mixture is easier to control because the composition of the liquid phase and the vapour phase are the same.
This is also an important concept in the case of mixed solvents with added extinguishing agent components, such as bromine, to control the flammability of the gas phase.
Copyright pirika.com since 1999-
Mail: yamahiroXpirika.com (Replace X with @.)
The subject line of the email should start with [pirika].
