2024.9.04
Chemistry at pirika.com > Chemistry > Chemical engineering > Reprint: gas-liquid equilibrium estimation by ASOG. > Chapter 1: Basic equations of solution theory > Chapter 2: ASOG method. > 2.3 Actual ASOG method calculation
2.3.6 Assessment of liquid-phase association using the ASOG method.
The fact that low-molecular-weight carboxylic acids also associate in the gas phase by forming some dimers was explained in 1.7 Handling of the association system. In that case, of course, association also occurs in the liquid phase. Low-molecular-weight carboxylic acids dimerise in the same molecule, but this is rather rare. In most cases, they associate in acid-base or donor-acceptor interactions. If the acid-base interaction is strong enough, it will precipitate as a salt. The tricky part is the donor-acceptor interaction. Let’s see this in action in the first 115-series example verification.
(95) Acetone_Chloroform
(101) Methyl Acetate_Chloroform
(114) Water_Formic Acid
Calculate the Acetone_Chloroform system using the ASOG method.
For example, the X-T diagram shows the following. As the mixing ratio is increased, the boiling point increases, which is higher than the boiling point of 56.3 °C for Acetone and 61.2 °C for Chloroform. The reason why the boiling point increases is that Acetone and Chloroform aggregate in the liquid phase, making it more difficult for them to evaporate.
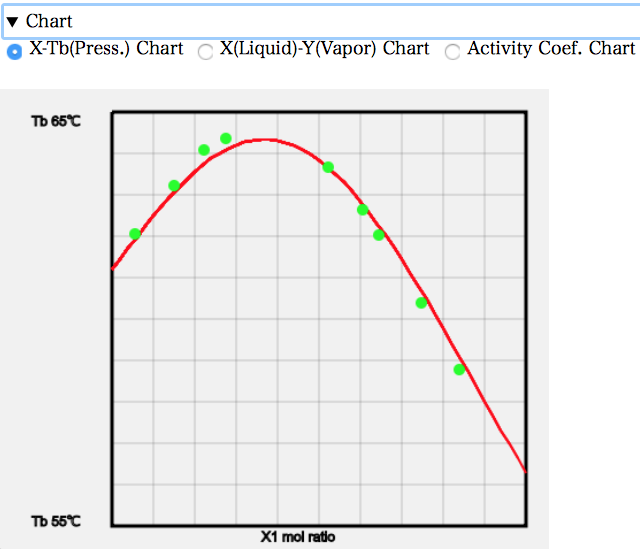
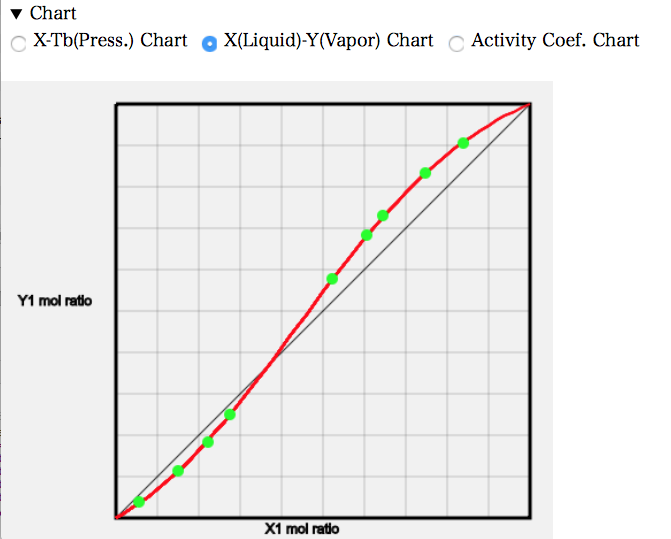
The X-Y diagram shows that on the left-hand side there is less Acetone in the composition of the gas phase than in the composition of the liquid phase. Since the original boiling point of Acetone is lower, the mole fraction of the gas phase is more in the other Acetone systems; the opposite is true because Acetone and Chloroform interact strongly. Approximately at a mole fraction of Acetone of 0.37, the composition of the liquid and gas phases coincide. This is the azeotrope point, which is called the highest azeotrope because the azeotrope temperature is higher.
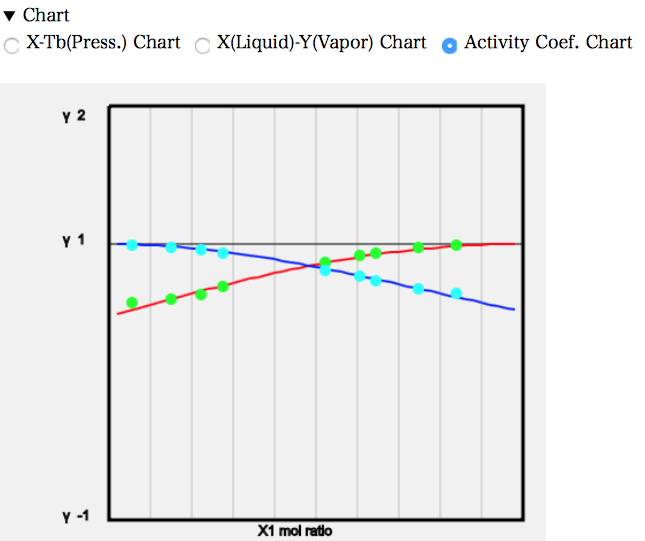
The activity coefficient diagram shows that the values are smaller than 1.
Considering the real solution version of Raoult’s law, P=P1*γ1*x1+P2*γ2*x2, γ1 and γ2 are smaller than 1, so P1 and P2 must be larger to match atmospheric pressure. Therefore, the temperature needs to be higher.
The experimental values are in good agreement with the calculated values of the ASOG method. It was mentioned earlier that the ASOG method in this publication does not reproduce the experimental values well, as the small molecule carboxylic acids aggregate in the gas phase. Low-molecular-weight carboxylic acids also aggregate in the liquid phase. Why can the liquid-phase aggregation system reproduce the experimental values?
The association of low-molecular-weight carboxylic acids is an association between the same molecules. The only groups in the system are the CH2 and COOH groups for acetic acid. Since the interglove interaction parameter akl between the same groups is assumed to be zero, ASOG considers only a(CH2,COOH), a(COOH,CH2). The entity causing the association is a(COOH,COOH), which is essentially non-zero. Since it is set to 0, the gas-phase association has to be taken into account.
The m,n parameters for the Acetone and Chloroform systems are output in the AiSOG Result, so let’s have a look. m,n can be used to calculate the group interaction parameter akl as follows.
ln akl = mkl + nkl⁄T
M[CO][CH2]=2.6172 N[CO][CH2]=-865.1
M[CH2][CO]=-1.7588 N[CH2][CO]=169.6
M[CCl3][CH2]=0.2352 N[CCl3][CH2]=-119.8
M[CH2][CCl3]=-0.1134 N[CH2][CCl3]=41.1
M[CCl3][CO]=-0.118 N[CCl3][CO]=-101.3
M[CO][CCl3]=0.3823 N[CO][CCl3]=3.1
This alone is difficult to understand, so let’s plot it against temperature.
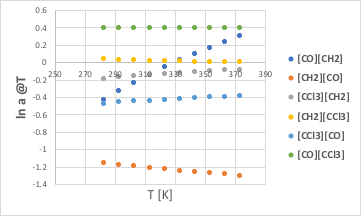
positive in the process, but the [CH2][CO] remains largely negative, so the CO/CH2 combination is always negative.
Since [CCl3][CH2] is always negative and [CH2][CCl3] is a small positive, the CCl3/CH2 combination is always negative.
[CCl3][CO] is always negative and [CO][CCl3] is always positive, but the absolute value of [CCl3][CO] is greater, so the CCl3/CO combination is also always negative.
(At first glance, the effect of CO/CH2 appears to be larger than that of CCl3/CO, but CCl3 has four heavy atoms and is therefore quadrupled.)
つまり、どのような比率で混合してもグループ活量係数はマイナスになる。ASOG法では、トータルの活量係数は ln γi = ln γiFH + ln γiG 式で計算する。グループ活量係数ln γiG がマイナスになっても、重原子の効果、ln γiFH は常にプラスになる。そこで、このようなトータルの活量係数ln γiがマイナスになるのは、小さな溶媒同士の混合物に限定される。In other words, the group activity coefficient is negative in any mixing ratio; in the ASOG method, the total activity coefficient is calculated by the equation ln γi = ln γiFH + ln γiG . Even if the group activity coefficient ln γiG is negative, the effect of heavy atoms, ln ln γiFH, is always positive. So, such a total activity coefficient ln γi becomes negative only for mixtures of small solvents.
Until now, little has been done to look closely at the group activity coefficient itself.
For acid-base interactions, you can get a rough idea by doing normal chemistry. However, not all donor-acceptor interactions, such as the CCl3/C=O interaction, have been quantitatively captured.
In fact, Ewell, who is well-known for his work on liquid-liquid extraction, considered this donor-acceptor interaction as a hydrogen bond, but only qualitatively.The application of such local activity coefficients will expand as the ASOG method becomes more parameterised.
For example, the introduction of a CCl3 group into the backbone of a drug is predicted to interact strongly with C=O and C(=O)O moieties in vivo. Is it possible to design a group that has a large negative local activity coefficient with a particular part of the organism?
The glass transition temperature of vinylidene chloride polymers (-CH2CCl2-) in food packaging materials increases significantly when copolymerised with methyl acrylate (-CC(COOCH3)-). How can we design polymers that increase gas barrier properties without chlorine?
ASOG provides an answer.
Copyright pirika.com since 1999-
Mail: yamahiroXpirika.com (Replace X with @)
The subject line of the email should start with [pirika].
