I exchanged emails with Dr Abbott, who is developing HSPiP with me, about the Pathway of skin permeation of compounds.
Originally, I was looking at the bioaccumulation factor of a compound with a self-organising neural network (SOM). I thought there was more than one HSP where compounds accumulate as logBCF because I could see areas of HSP where things like dioxin gather and areas where steroids gather, so I just tried to do the same thing with skin.
By the way, it is rather pitiful that rabbits are so often used in animal experiments.
Rabbits, in particular, are often investigated not only for oral and dermal toxicity, but also for irritation of the eyes.
It would be good if alternative methods to animal testing could be established soon.
In animal experiments, toxicity varies according to animal species. This is difficult because toxicity may be low in rats and rabbits but high in monkeys and pigs.
The HSPiP currently has a database of more than 10,000 compounds and has accumulated LD50 information.
The most common oral LD50 is for rats and the most common one for skin is for rabbits.
We retrieved from the database those with LD50(oral, Rat)<180.
The toxicities of both orals, rats and skins rabbits were extracted as follows.
| CAS | name | LD50(oral,Rat) | LD50(skin,rabbit) | logP | logS |
| 75-86-5 | acetone cyanohydrin | 18.65 | 15.8 | -0.03 | 2.00 |
| 107-19-7 | propargyl alcohol | 20 | 16 | -0.38 | 2.00 |
| 107-11-9 | allylamine | 102 | 35 | 0.03 | 2.00 |
| 107-18-6 | allyl alcohol | 64 | 45 | 0.17 | 2.00 |
| 107-07-3 | 2-chloroethanol | 71 | 67 | 0.03 | 2.00 |
| 1464-53-5 | Butadienedioxide | 78 | 89 | -0.28 | 2.00 |
| 111-44-4 | Di-(2-Chloroethyl) Ether | 75 | 90 | 1.29 | 0.24 |
| Average | 0.12 | 1.75 |
Most compounds have a logS (solubility in water g/100g) of 2, so they are completely water soluble.
The logP (octanol/water partition ratio) is also found to be very small.
I believe that a condition for the onset of toxicity of any toxic substance is that it must penetrate a boundary and become highly concentrated in the blood.
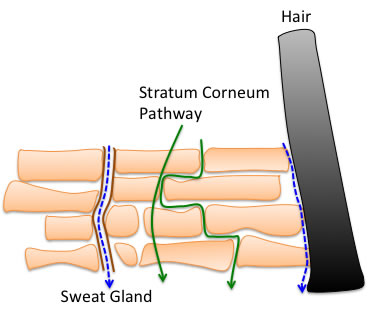
If a water-soluble compound is more toxic when applied to rabbit skin, it is not surprising that there is a Pathway somewhere in the skin that allows water-soluble compounds to penetrate.
Whether that is a hydrophilic membrane without holes, or holes that release sweat, or holes along the hair follicles, I do not know.
These would be pathways where the δH, hydrogen bonding term out of the HSP would come into play, especially as they are compounds such as alcohols.
Next, I extracted those that were highly toxic for orals and rats, but less toxic for skins rabbits.
| CAS | name | LD50(oral,Rat) | LD50(skin,rabbit) | logP | logS |
| 591-87-7 | allyl acetate | 130 | 1021 | 0.97 | 0.31 |
| 109-75-1 | vinylacetonitrile | 115 | 1410 | 0.40 | 0.53 |
| 13952-84-6 | sec-butylamine | 152 | 2500 | 0.74 | 1.05 |
| 79-22-1 | methyl chloroformate | 60 | 7120 | 0.14 | 0.97 |
| 126-98-7 | methacrylonitrile | 120 | 12500 | 0.68 | 0.41 |
| Average | 0.59 | 0.65 |
Such compounds are less water soluble and have a greater logKow.
The term ‘fat-soluble’ is a bit of a misnomer, but fat-soluble compounds are highly toxic when absorbed through the stomach or intestines and reach high blood levels, but when applied to rabbit skin, they do not have pathways or have low toxicity.
Later, Skin rabbits are highly toxic while Oral rats are less toxic.
| CAS# | name | LD50(Oral Rat) | LD50(SkinRabbit) | logP | logS |
| 91-17-8 | Decalin | 4170 | 6 | 4.2 | -4.05 |
| 140-67-0 | Benzene, 1-methoxy-4-(2-popenyl)- | 1230 | 5 | 3.47 | -1.75 |
| 3680-02-2 | Methyl Vinyl Sulfone | 570 | 32 | -0.75 | 1.69 |
| 930-68-7 | 2-cyclohexenone | 220 | 70 | 0.61 | 0.56 |
| 107-14-2 | Chloroacetonitrile | 220 | 71 | 0.45 | 1.00 |
| Ave. | 1.596 | -0.51 |
The logP and logS in this case vary widely.
It is not clear to me what is meant by the fact that it is not easily absorbed from the stomach and intestines, but from the skin it migrates into the bloodstream and becomes toxic.
All I can say is that using the HSP of these compounds to draw a toxicity SOM is a tremendous food for thought.
There are a limited number of compounds for which LD50s exist for both animals, but there are a whole lot more compounds for which there is data for just one of them.
It would be good if experts in this field could use SOM to analyse the results and lead to a reduction in animal testing.
