Learn to program with your child.
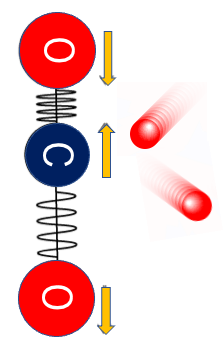
Carbon dioxide gas absorbs infrared light and sends some of it back to the earth because of its inverse symmetric stretching vibrations, while nitrogen and oxygen are diatomic molecules, so they cannot vibrate in an inverse symmetric stretching manner and therefore do not absorb infrared light.
What kind of parents would teach their children that?
First, press the Start button.
| CO2 Concentration ppm |
|---|
The sun is on the left.
On the right is the earth.
The blue ball comes from the sun. The blue sphere is a high-energy light coming from the sun.
When it reaches the right earth, it reflects back as a red-colored ball. This light is called far-infrared light, which has weak energy but high warming power.
You may have heard of far-infrared radiation because some heaters and other devices are labeled as such.
In the screen, there is carbon dioxide gas CO2.
The blue sphere of carbon dioxide gas slips through.
The red sphere is reflected back.
If the energy going in is balanced by the energy going out, the earth’s temperature will not change.
However, since CO2 beats back far-infrared radiation, the far-infrared effect accumulates and the earth is heated.
Let’s simulate what happens when this CO2 increases. How to make the slider was explained in No2-3-1. (Only for Japanese)
As the CO2 concentration reaches about 400 ppm, the sphere becomes more and more confined to the area on the right.
And if you want to learn while playing, press the arrows on your keyboard.
Now, try your best to move the arrows and see the ball bounce back and forth.
It is fun to learn the basics of programming by changing the speed and number of balls.
It is open to parents and children in chemistry and pharmacy for fun and programming. (Only Japanese)
