Did you do the homework I gave you in the first part?
There will always be a big difference between those who have done the calculations and felt the difference, and those who feel they understand without doing the calculations.
| Mol Volume | SO4Na-Agg# | Micelle-Volume | Micelle-radius | Micelle-Surface | Surface/Agg# | |
| C6H13 | 120.0 | 17 | 2040.3 | 7.9 | 777.8 | 45.8 |
| C7H15 | 136.7 | 22 | 3008.0 | 9.0 | 1007.5 | 45.8 |
| C8H17 | 153.4 | 27 | 4141.6 | 10.0 | 1247.0 | 46.2 |
| C9H19 | 169.2 | 33 | 5582.8 | 11.0 | 1521.6 | 46.1 |
| C10H21 | 185.9 | 50 | 9295.6 | 13.0 | 2137.6 | 42.8 |
| C11H23 | 202.6 | 45 | 9118.0 | 13.0 | 2110.3 | 46.9 |
| C12H25 | 219.3 | 62 | 13595.6 | 14.8 | 2754.3 | 44.4 |
First, it can be seen that the surface area of the micelle occupied by each hydrophilic part of the surfactant hardly changes, averaging 45.4 Å2, even though the size of the hydrophobic field changes.
| hydrophilic part | Hydration Area Å2 |
| SO4Na | 45.4 |
| COOK | 46.5 |
| COONa | 47.3 |
| SO3Na | 44.8 |
| NH3Cl | 48.3 |
| NM23Cl | 48.2 |
| NMe3Br | 47.6 |
| Pyridine-Br | 47.3 |
| Pyridine-Cl | 65.9 |
| Phenyl-SO3Na | 80.8 |
It can also be seen that the surface area on the micelle occupied by each hydrophilic part of the surfactant, whether anionic or cationic, hardly changes when the size of the hydrophobic field changes.
Pyridine hydrochloride and sodium aromatic sulfonate both have a very large hydration area.
The sodium salt of aromatic sulfonate foams well even in very small amounts, but it is not biodegradable and is rarely used from an environmental point of view.
This property is thought to be based on the acid dissociation constant.
Carboxylic acids in hydrocarbon systems are weak acids. However, perfluorinated carboxylic acids are strong acids, and their salts foam so well that they were used in fire extinguishers. (But this is no longer used, as it is also known as the PFOS problem.)
Similarly, phenolic OH has acidity (O–) and is easily hydrated.
Let’s calculate how many water molecules are coordinated to the phenolic OH.
The assumption is that in HPLC systems using ODS columns, the retention time (RT) correlates very well with,
tot HSP/Mol Volume
HPLC Retention Time of Carboxylic Acids
Therefore, if we use Y-MB in HSPiP, we can get [dD, dP, dH] and MolVolume, so we can compare with retention time immediately.
Let’s use this correlation to do some actual calculations.
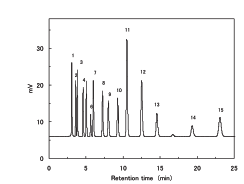
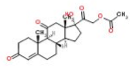
A Japanese National Institute of Health report (Bull. Natl. Inst. Health Sci., 127, 54-61 (2009)) titled “Simultaneous collection test on steroid combination in cosmetics claiming to be effective for atopic dermatitis.
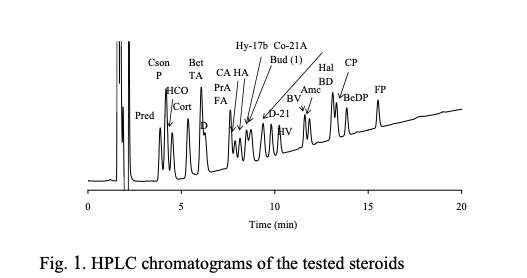
Let’s copy and paste this data into Excel and draw a graph.
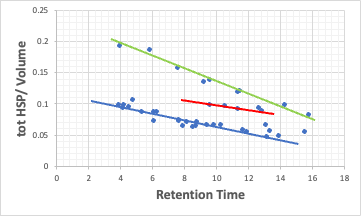
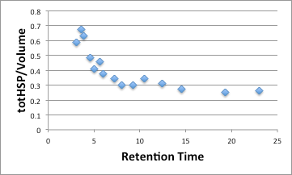
Unlike the case of carboxylic acid, three lines appear roughly.
The retention time of parabens used as preservatives is too large for the value of tot HSP/Volume. Also, some of the steroids ride the red line.
Where does this difference come from?
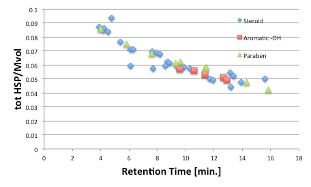
If we assume that 10 water molecules are coordinated to the phenol part, and we add 18 cm3*10 molecules = 180 to the molecular volume, then the three lines become one.
This is a very common occurrence in HPLC analysis.
In the HPLC of UV absorbers, two lines appear when plotted in the same way, one with phenolic hydroxyl groups and the other without, and when corrected with 10 waters, the anomaly disappears.
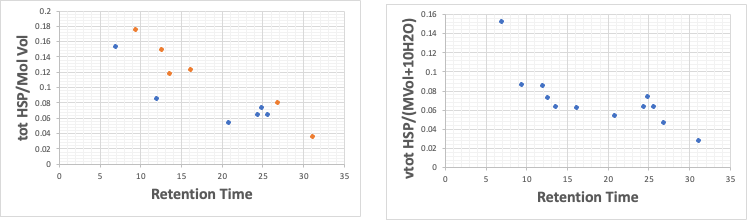
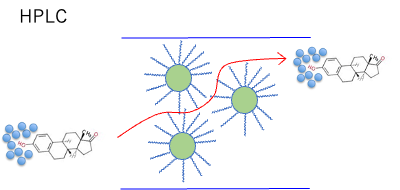
In other words, the water that hydrates to the phenolic hydroxyl group is probably bound so tightly that the size of the molecule is always kept large, even if it is dynamically replaced.
The same is true for flow through the bloodstream.
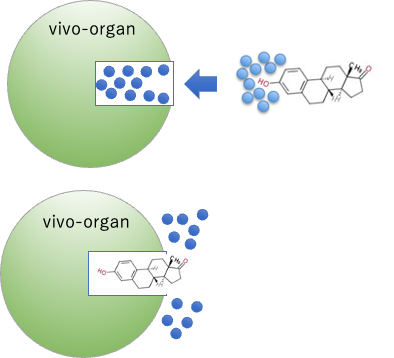
The keyhole of a living organ is filled with water. Therefore, the dielectric constant is 87.74.
When a substrate enters there, it pushes out the hydrated water, the water that was in the keyhole.
Therefore, the keyhole becomes a non-polar space with a dielectric constant of about 4.
This is the essence of how organisms are able to use hydrophilic and hydrophobic reactions.
It is thought that amide groups and others are also hydrated to some extent.
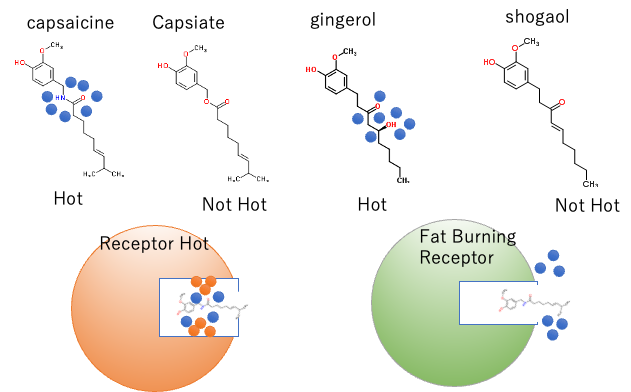
For example, the following compounds are known as pungent compounds. All of them have a fat-burning effect. Capsaicin is very pungent, but capsiate, developed by Ajinomoto, is not pungent but has a high fat-burning effect. The difference is in the amide and ester groups: Gingerol is the pungent component of ginger, while Shogaol, which is produced by heating, is less pungent. The difference is that Gingerol has a hydroxyl group that hydrates like capsaicin.
Once the hydrated water is removed, the shape is almost the same, so it responds to fat burning receptors in the same way. However, it is easier to understand if we consider that the receptors that recognize pungency cannot match the shape without the hydrated water.
Incidentally, House Foods(Japanese company) has patented the use of cyclodextrin to eliminate the pungency of capsaicin.
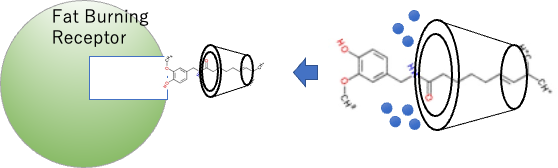
When capsaicin is encapsulated in cyclodextrin, the hydration water of the amide group is removed, so the spicy receptors cannot recognize it. The capsaicin is then sent to fat-burning receptors. It’s a diet drink for people who don’t like spicy food, but want to lose weight.
If you are going to do drug delivery systems (DDS), you may find it interesting to review the concept of hydration carefully.
By the way, I love to drink. What will happen if ethanol is added to the hydration structure?
The hydration structure will break down, the polarity will change drastically, and the molecular volume may also change due to the decrease in hydration water.
I hope it will give me a reason to drink.