I’m not sure what they call the role, but it’s not clear to me either.
The program will decide if it should be included in the QSAR formula.
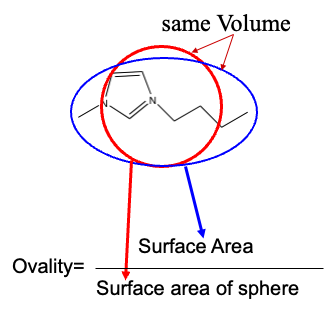
First, the definition of Ovality.
This was included in the old ChemDraw3D. It was useful for predicting the physical properties of ionic liquids.
First, it assumes that when you have a molecule, you can get the volume of the molecule (NW/Density) and the surface area of the molecule.
I have the molecular volume and molecular surface area from COSMO, since the values in the database are calculated by MOPAC.
The volume of the molecule is to use the experimental value (MW/Density). Using the molecular orbital method for this is not a good thing in my experience.
The molecular surface area can only be brought from the molecular orbital calculation in reverse.
It cannot be obtained from experimental values.
This calculated value becomes the numerator.
Then consider a true sphere that has exactly the same volume as the molecular volume of this molecule.
The true sphere has the smallest surface area in the same volume.
Bring this to the denominator.
So, if the shape of the molecule is close to a sphere, the Ovality will be 1. (It can be less than 1 due to the discrepancy between the experimental and MO calculations.) In the case of ionic liquids, there was a high correlation between melting point and Ovality.
Imaginatively speaking, a large soft squishy molecule can stretch out (Ovality=2) or curl up like a piece of lint (Ovality=1), but one that originally has an Ovality of 1 becomes a stiff, shape-changing molecule. (However, polycyclic compounds also cannot change shape.)
The influence of these shape factors on solubility is not clear.
Therefore, I will try to understand the meaning of Ovality by correlating it with various physical properties.
Entropy of melting (heat of fusion/ melting point) correlates with Ovality.
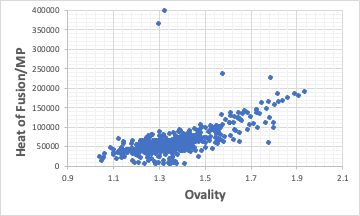
The heat of fusion divided by the melting point is highly correlated with Ovality.
The closer the molecule is to a spherical shape, the smaller the heat of fusion becomes.
Therefore, when heat is applied to a solid of spherical molecules, we can see the sublimation phenomenon in which the molecule becomes a gas instead of a liquid. So this will be easy to accept.
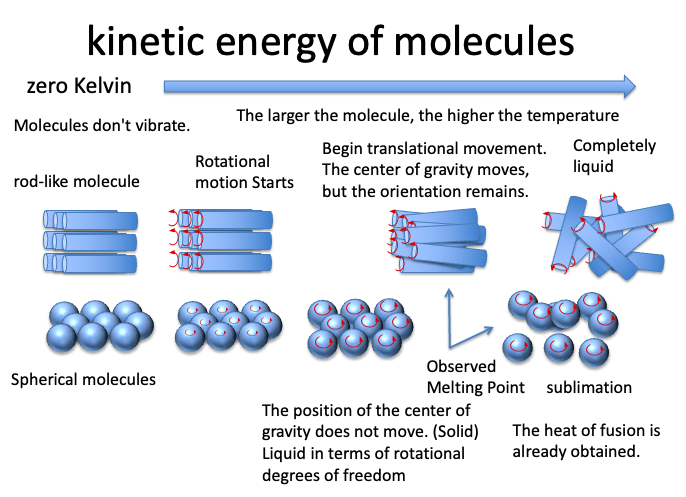
This leaflet is from a lecture I gave at the University of Tokyo “Chemistry of Molecular Assemblies”.
To be precise, there is a problem with the way the melting point is taken.
A spherical molecule is considered solid because the position of its center of gravity does not change as it heats up and moves more and more.
However, in terms of rotational degrees of freedom, they are liquid.
By the time the position of the center of gravity begins to move and is observed as the melting point, the heat of fusion is very small because enough kinetic energy has already been obtained.
It may seem strange that Ovality is an indicator that has nothing to do with the size of molecules.
However, for Ovality to be large, the molecules must also be large.
Correlation between molecular density and Ovality
Taking the density of molecules at the boiling point in kmol/m3 and examining the correlation with Ovality, we find the following. The closer the molecule is to a sphere, the larger the number of moles per m3.
The same correlation is obtained at 25°C, but much more ambiguous.
Considering the densest packing structure when shaken at high temperatures, we can imagine that the most can be packed when Ovality=1.
Therefore, density and molecular volume (molecular weight/density) at the boiling point are very important indicators for estimating physical properties.
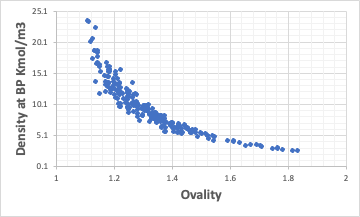
It would be natural that the larger the molecule, the smaller the number of moles per m3.
However, if we change the horizontal axis to molecular weight, we get the following.
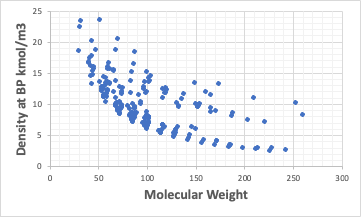
There are heavier atoms, such as halogen atoms, which make it difficult to see correlations.
Critical temperature/boiling point correlates with Ovality.
It is known that the critical temperature/boiling point is approximately 1.5.
Therefore, the critical temperature of a compound whose critical temperature is unknown is often set to 1.5 times the boiling point (taken in Kelvin units).
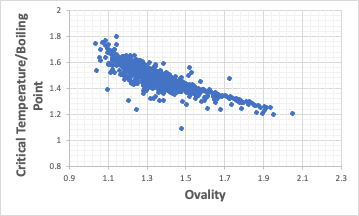
The correlation between critical temperature/boiling point and Ovality is shown in the figure above. From a practical point of view, even for compounds with unknown critical temperatures, the critical temperature can be more accurately estimated by calculating Ovality.
However, the scientific implications are difficult to make sense of.
Molecules of the same molecular weight of the same family have lower boiling points the closer they are to a spherical shape.
It is thought that this is because there are fewer contact surfaces between molecules.
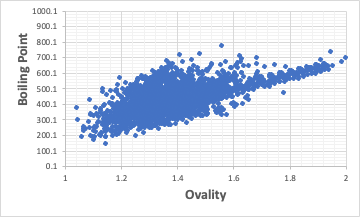
If the critical temperature is similarly lowered, then the critical temperature/boiling point should be constant.
If Ovality is close to 1 and the critical temperature/boiling point is greater, then it indicates that the critical temperature does not decrease as much as the boiling point decreases.
Why that is, to me, is still not clear.
Relationship between Thermal Conductivity and Ovality
Molecules with Ovality close to 1 cannot take a large conformational change. Then, when considering molecular collision, it becomes closer to rigid sphere collision, and I feel that the loss of heat conduction becomes smaller.
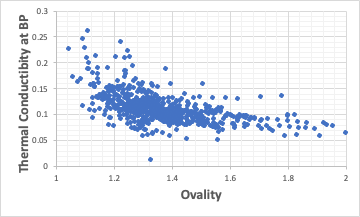
There seems to be a general trend, but it’s not very clear.。
Ovality of the repeating units that make up the polymer
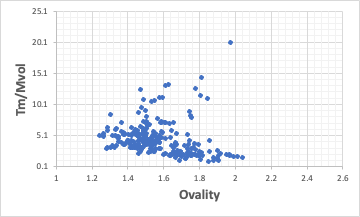
The melting point of the polymer divided by the molecular volume of the repeat unit correlates with the Ovality of the repeat unit.
However, the relationship is inevitably ambiguous. This is because in the case of radical polymerizable polymers, the units are clearly defined by small monomer units, but in the case of polyesters and polyamides, the repeating units themselves can be very large.
I can’t say anything in conclusion.
How does Ovality contribute to polymer solubility? I can’t answer this question.
But if you dig deeper, you may find a very interesting vein.
After all, it is a parameter that is often selected in QSAR.
