2024.9.04
pirika.comで化学 > 化学全般 > 化学工学 > 復刻版:ASOGによる気液平衡推算法 > 第1章 溶液論の基礎式
1.7 会合系の取り扱い
酢酸、プロピオン酸などの低分子のカルボン酸などは、気相で一部が会合する。その本質はカルボキシル基の2分子間水素結合だ。通常の水素結合は、2次元、3次元に広がる。しかし、カルボキシル基の場合には、ヘテロ原子(酸素や窒素)についた水素と、その水素を受け止めるカルボニル酸素の両方を近い距離に持つので、ダイマーを作って安定化してしまう。
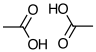
カルボキシル基を含む成分Aとそれ以外のBからなる2成分では,次のような同種分子間会合と異種分子間会合が考えられる.
2A1 ⇆ A2, 2B1⇆ B2 (1-13)
A1+B1 ⇆ AB (1-14)
A1, B1は成分AおよびBのモノマー,A2,B2はAおよびBのダイマー,またABはAとBの異種会合分子を示している.
この会合反応の、平衡定数がわかっているものは、ギ酸、酢酸、プロピオン酸の3つしかない。異種分子間会合の平衡定数がわかっているものはないので、実質的には、2A1 ⇆ A2だけを考えれば良いことになる。
平衡定数をKAとおくと、次の式が成立する。
KA=[A2 ] / [A1 ]2 (1-15)
[ ]は濃度を示す。
ある温度、圧力、体積が決まった時に、気相中に何モル(何個)の分子がいるかは1つに決まる。その時には、ダイマーであっても1つの分子と数える。
そこで[A1]+ [A2]=1になる。
KA=(1-[A1 ])/[A1 ]2 (1-16)
これをA1について解くと、次のようになる。
[A1 ]=(-1+√(1+4KA ))/2KA (1-17)
もし、A成分もB成分もカルボキシル基を持つのであれば、Bについても同じことを行う。
そして、異種分子間会合の平衡定数はKAB=2√(KA KB )を使う。
このようにして、濃度に補正をかけた上で、気液平衡の計算を行う。
次節:1.8 活量、活量係数の入手方法
Copyright pirika.com since 1999-
Mail: yamahiroXpirika.com (Xを@に置き換えてください)
メールの件名は[pirika]で始めてください。
