2024.9.04
Chemistry at pirika.com > Chemistry > Chemical engineering > Reprint: gas-liquid equilibrium estimation by ASOG. > Chapter 1: Basic equations of solution theory > Chapter 2: ASOG method. >
2.2 Estimation of the gas-liquid equilibrium using the ASOG method
In many cases, vapour-liquid equilibrium measurements are made at constant pressure and varying mole fractions to determine the temperature at which a constant pressure is reached. The pressure is often atmospheric, but in the case of compounds with a high boiling point, the pressure may be reduced. In other cases, the change in pressure is measured by changing the mole fraction at a constant temperature. In this way, the mole fraction of the gas phase and the temperature are determined in relation to the mole fraction of the liquid phase at constant pressure. Alternatively, the determination of the mole fraction of the gas phase and the pressure with respect to the mole fraction of the liquid phase at constant temperature is done experimentally by measuring the vapour-liquid equilibrium and by computer-based estimation of the vapour-liquid equilibrium.
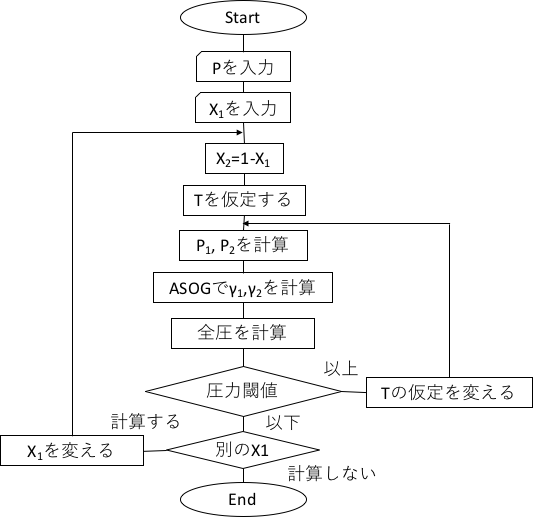
The activity coefficient γi is a function of the mole fraction xi and temperature. The mole fraction xi is specified from 0 to 1.0, e.g. in increments of 0.02. A mole fraction of 0 means that the first component is 0 and therefore pure water, while a mole fraction of 1 means pure ethanol. (In the world of vapour-liquid equilibrium, the lower boiling point is conventionally the first component.) Pure ethanol and water need not be calculated, so the mole fraction xi is calculated from 0.02. Once the mole fraction is determined, the group fraction Xl (fraction of groups present in the system) of group l in the liquid phase is determined. With a mole fraction of 0.02, there is very little ethanol, so XCH2 and XOH will have very small values. Once this group fraction Xl and the group interaction parameter akl are determined, the group activity coefficient Γk of group k is determined.
The problem here is the temperature. Even if mkl and nkl out of the group interaction parameters ln akl = mkl + nkl⁄T are taken from the database, akl cannot be obtained unless the temperature T(K) is determined. Therefore, the group activity coefficient Γk = function (xj, T) in the programme. This means that the group activity coefficient is determined when the mole fraction and temperature are specified. How do we actually determine the temperature T? As we did in 1.1, we calculate the activity coefficient γi by assuming 100 (water boiling point)(1-X1) + 78.2 (ethanol boiling point)X1 and an initial value of temperature. And the real solution version of Raoult’s law,
P=P1*γ1*x1+P2*γ2*x2
to calculate the total pressure. If the calculated value is greater than atmospheric pressure, the temperature T is reduced, if it is less than atmospheric pressure, the temperature T is increased and a temperature T equal to atmospheric pressure is found by convergence calculation. If this is calculated over the entire composition range, the gas-liquid equilibrium has been estimated. To calculate the vapour pressure of a pure component at a certain temperature, the vapour pressure equation of the pure component is required. This is also a physical property value that can be determined by selecting a component, so the Antoine constant is included in the database of pure components.
The flowchart of the ASOG method estimation for constant pressure is shown below.
vj^FH とvkiとAntoine定数は純成分データベースに登録する。
グルーブ間相互作用パラメータaklはASOGパラメータデータベースに登録する。
蒸気圧式のAntoine A, B, Cは純成分データベースに登録する。
それさえできていれば、任意の組み合わせで、最初に行いたかった事ができるようになる。
(本来であれば、ギ酸、酢酸などは気相会合するとして扱わなくてはならないが本書のバージョンでは対応していない。)
The vjFH and vki and Antoine constants are registered in the pure component database.
The group interaction parameter akl is registered in the ASOG parameter database.
The vapour pressure equations Antoine A, B and C are registered in the pure component database.
Once that is done, you can use any combination to do what you wanted to do in the first place.
(Originally, formic acid, acetic acid, etc., had to be treated as gas-phase associations, but this is not supported in this version of the book.)
It is possible to predict the boiling point and gas-phase composition of an arbitrary mixture of solvents when the composition changes.
This is because the ASOG method makes it possible to know the activity coefficient of any given solvent pair at a given mixing ratio and at a given temperature.
Next section: 2.3 Actual ASOG method calculations
Copyright pirika.com since 1999-
Mail: yamahiroXpirika.com (Replace X with @.)
The subject line of the email should start with [pirika].
