2024.9.04
pirika.comで化学 > 化学全般 > 化学工学 > 復刻版:ASOGによる気液平衡推算法 > 第2章 ASOG法 > 2.3 実際のASOG法計算
2.3.6 ASOG法による液相会合の評価
低分子のカルボン酸が気相でも一部ダイマーを作って会合していることは1.7 会合系の取り扱いで説明した。その際には、当然、液相でも会合が起きている。低分子のカルボン酸は同一分子でダイマー化するが、これはむしろ稀なことだ。多くの場合は、酸-塩基、もしくはドナー-アクセプター相互作用で会合する。酸-塩基の相互作用は強ければ塩として析出することになる。厄介なのは、ドナー-アクセプター相互作用だ。最初の115系例題検証で実際に見てみよう。
(95) Acetone_Chloroform
(101) Methyl Acetate_Chloroform
(114) Water_Formic Acid
Acetone_Chloroform系をASOG法で計算する。
例えば、X-T線図を見ると次のようになる。混合比率を増やしていくと、沸点が上昇していくのが分かる。Acetoneの沸点56.3℃、Chloroformの沸点61.2℃よりも高い沸点が必要になる。何故沸点が上昇するのかというと、液相中でAcetoneとChloroformが会合を起こして蒸発しにくくなっているからだ。
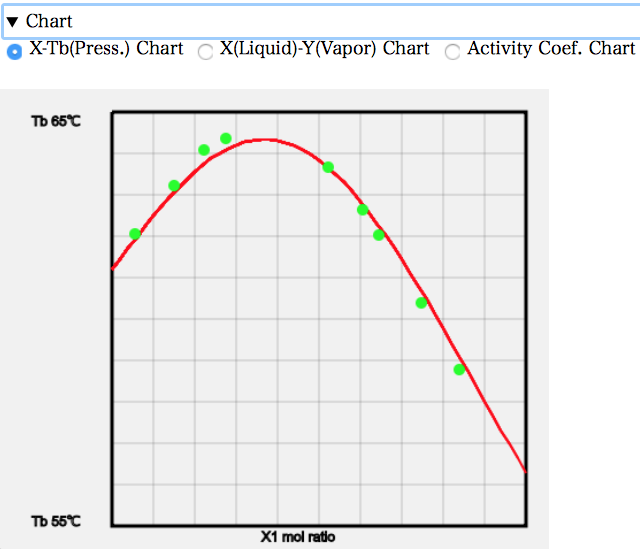
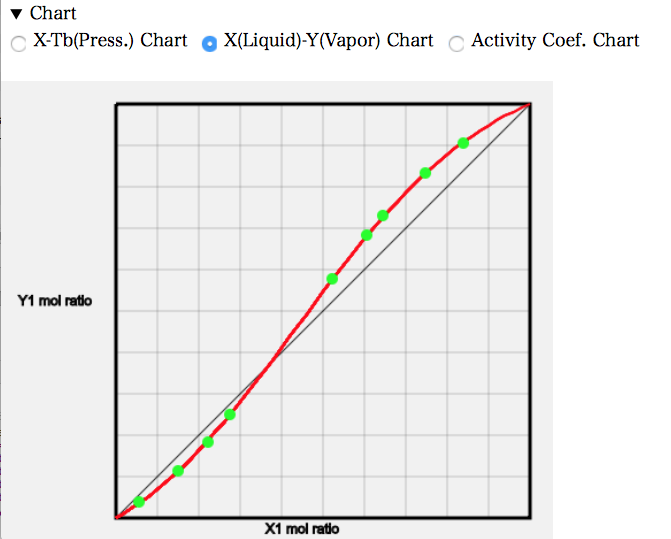
X-Y線図を見ると、左端では液相の組成よりも気相の組成でAcetoneが少なくなっている。元々の沸点はAcetoneの方が低いので、他のAcetoneの系では気相のモル分率は多くなった。AcetoneとChloroformが強く相互作用するので逆になってしまう。およそ、Acetoneのモル分率が0.37あたりで液相組成と気相組成が一致する。そこが共沸点だが、共沸温度が高くなるので最高共沸と呼ぶ。
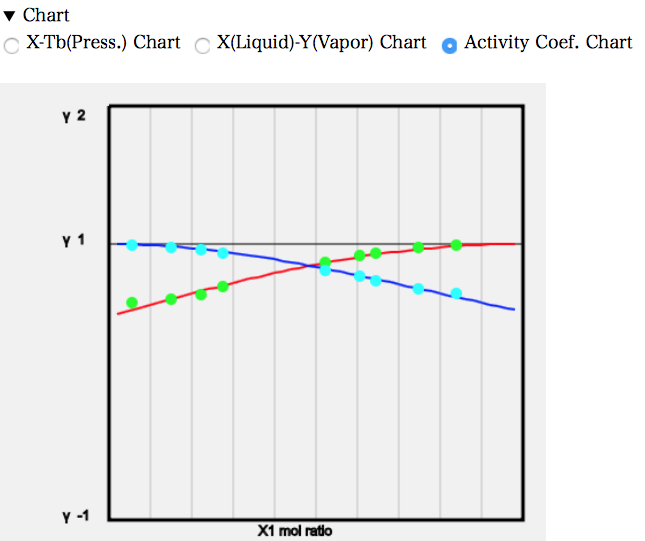
活量係数線図を見ると、値は1より小さくなる。
ラウールの法則の実在溶液版の式、P=P1*γ1*x1+P2*γ2*x2で考えると、γ1、γ2が1より小さいわけだから、P1、P2はもっと大きな値にならないと大気圧に一致しない。そこで温度はより高くなる必要がある。
実験値はASOG法の計算値とよく一致している。先の低分子のカルボン酸は気相会合するので、本書のASOG法では実験値をうまく再現できないと述べた。低分子のカルボン酸も液相で会合を起こしている。何故、液相会合系は実験値を再現できてしまうのだろうか?
低分子のカルボン酸の会合は、同じ分子同士の会合だ。系の中にあるグループは、酢酸の場合、CH2グループとCOOHグループだけだ。同じグループ同士のグルーブ間相互作用パラメータaklは0とおくから、ASOGはa(CH2,COOH), a(COOH,CH2),だけを考える。会合を起こしている主体は、a(COOH,COOH)で、本来これは0ではない。それを0と置いてしまっているので、気相会合を考慮に入れなくてはならなくなる。
AcetoneとChloroform系のm,nパラメータはAiSOG Resultに出力されているので、みてみよう。m,nがわかれば次式でグルーブ間相互作用パラメータaklが計算できる。
ln akl = mkl + nkl⁄T
M[CO][CH2]=2.6172 N[CO][CH2]=-865.1
M[CH2][CO]=-1.7588 N[CH2][CO]=169.6
M[CCl3][CH2]=0.2352 N[CCl3][CH2]=-119.8
M[CH2][CCl3]=-0.1134 N[CH2][CCl3]=41.1
M[CCl3][CO]=-0.118 N[CCl3][CO]=-101.3
M[CO][CCl3]=0.3823 N[CO][CCl3]=3.1
これだけだと分かりにくいので、温度に対してプロットしてみる。
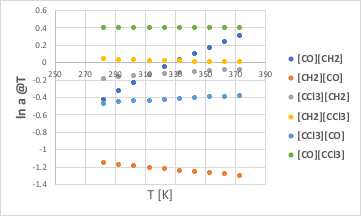
[CO][CH2]は途中でマイナスからプラスになるが、[CH2][CO]は大きくマイナスのままなので、CO/CH2の組みは常にマイナスになる。
[CCl3][CH2]は常にマイナスで、[CH2][CCl3]は小さなプラスなので、CCl3/CH2の組みは常にマイナスになる。
[CCl3][CO]は常にマイナス、[CO][CCl3]は常にプラスだが、絶対値が[CCl3][CO]の方が大きいので、CCl3/COの組みも常にマイナスになる。
(一見、CO/CH2の効果の方が、CCl3/COよりも大きそうに見えるが、CCl3は重原子が4つなので4倍される。)
つまり、どのような比率で混合してもグループ活量係数はマイナスになる。ASOG法では、トータルの活量係数は ln γi = ln γiFH + ln γiG 式で計算する。グループ活量係数ln γiG がマイナスになっても、重原子の効果、ln γiFH は常にプラスになる。そこで、このようなトータルの活量係数ln γiがマイナスになるのは、小さな溶媒同士の混合物に限定される。
これまで、グループ活量係数自体を細かく見ることは、あまりなされて来なかった。
酸-塩基相互作用であれば、普通に化学をやってくれば、おおよそ見当はつく。しかし、CCl3/C=Oの相互作用のような、全てのドナー-アクセプター相互作用が定量的に捕らえられているとは言えない状況だ。
実は液液抽出で著名なEwellはこのドナー-アクセプター相互作用を水素結合として捉えてはいたが、あくまでも定性的な捉えかただった。ASOG法のパラメータ化が進むとこうした局所活量係数の応用は広がると思う。
例えば、ある薬剤の骨格にCCl3基を導入すると、生体内のC=OやC(=O)Oの部分と強く相互作用することが予測される。生体のある特定部位と局所活量係数が大きくマイナスになるグループが設計できないか?
食品包装材の塩化ビニリデンポリマー(-CH2CCl2-)はアクリル酸メチル(-CC(COOCH3)-)と共重合するとガラス転移温度が大きく上昇する。塩素を使わずに、ガスバリアー性を上げるポリマー設計はどうしたら良いだろうか?
ASOGは答えてくれる。
Copyright pirika.com since 1999-
Mail: yamahiroXpirika.com (Xを@に置き換えてください)
メールの件名は[pirika]で始めてください。
