2024.9.04
pirika.comで化学 > 化学全般 > 化学工学 > 復刻版:ASOGによる気液平衡推算法
第3章 ASOG法の問題点
これまで見てきたように、ASOG法で気液平衡を推算したり、計算された活量係数を利用するのは非常に簡単な事だ。溶媒を選んで(人間がやる)、パラメータをデータベースから選び出して(コンピュータがやってくれる)、組成を変化させた時の温度を収束計算して(コンピュータがやってくれる)結果を出力するだけだ。もちろん、溶媒ペアを次々に変えながら一連の計算を行い、例えば共沸があれば、共沸組成と温度の推算値を全てリストアップするぐらいの事はコンピュータの得意とするところだ。
ただし、適用できるのは、次の2点ができている場合だけだ。
vjFH とvkiとAntoine定数は純成分データベースに登録されている。
グルーブ間相互作用パラメータaklはASOGパラメータデータベースに登録されている。
本質的に難しいのは、2番目ののグルーブ間相互作用パラメータが求まっているかという問題だ。自分の知りたい溶媒、例えばフロン代替化合物に含まれるグループ(CF3など)がデータベースに登録されていない場合には、計算ができない(もしくは、akl=0と無視される)事だ。
また、ASOG法は解析的グループ溶液理論の1種だ。グループ溶液理論によれば,「ある分子中のひとつのグルーブの寄与はその分子中の他のグルーブの寄与に独立であると仮定し,加成性の原理を適用できる」ことを前提にしている.「他のグループの寄与に独立で無い」場合も実際には存在し、加成性が成り立たないこともある。
vjFH は重原子数なので簡単だが、vkiの数え方に関しては検討が十分ではない。
CH3-, -CH2-, >CH-, >C<は1:1:0.8:0.5個という比は小島・栃木が解析し、最適な値として求めたものだ。他のグループについてもいろいろ応用できるはずのものだが、実際には、水の数え方を1.6にする以外には使われていない。
例えば、環状の構造に導入された2重結合、芳香族についた2重結合、ニトリルについた2重結合の数え方が一般化されれば、さらに精度は向上すると思われるが、そうした研究は進んではいない。
この2つの問題は、ASOG法のパラメータ決定法の問題と言える。様々な書籍を調べても、ASOG法のパラメータ決定法に関してはほとんど記載がない。実験値と推算値の誤差が最小になるように、シンプレックス法、マルカート法などを使ってパラメータを決定したとある程度だ。実際のパラメータ決定法を栃木先生にお伺いしたところ、「パラメータ決定は、(UNIFAC法で著名な)Gmeling先生のところに出向いて一括して決定している」とのことだった。「ごく限られた数人の専門家が特殊な環境でパラメータを決定」するしかないのがASOG法の最大の問題点になる。
また、Antoine定数にも問題点がある。
ASOG法のパラメータを決定する場合には、様々な論文、データベースから精度の高い気液平衡の測定値を収集する。そうした論文中には、測定した系の純物質のAntoine定数が記載されている。ところが、そうしたAntoine定数が何時も一定とは限らない。
筆者が収集した気液平衡のデータの中にある、エタノールのAntoine定数の値をまとめると、次のような表になる。
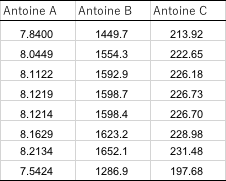
同じエタノールでも、様々なAntoine定数が使われている事がわかるだろう。実測の気液平衡のデータから計算した活量係数はその時に使ったAntoine定数で無いと再現できない。ASOG法で推算するエタノールを含んだ系はどの値を使うのが正しいのだろうか?
水のAntoine定数なども、様々な値が公開されている。本来、Antoine定数は蒸気圧測定の温度範囲が指定されているものだ。
0℃から沸点100℃までの蒸気圧データを解析した時にはAntoine定数はいくつというように。
50℃から150℃のデータを解析した時にはいくつ、と決まるものだ。
エタノールとの気液平衡を測定する相手方の沸点次第で、Antoine定数の実験値は変わって当然のものだ。しかし純成分の分子構造だけからASOG法を使おうとするとAntoine定数は1つに決まっていないと困るという問題点がどうしても残る。書籍や論文中に記載の精度と比べ固定値のASOG法を使った場合は、どうしても精度が落ちることはご理解していただきたい。それがキーマテリアルならデータベースの値を変更すればいいだけだ。
グループ間の独立性の問題はこれまでにも認識はされていたのだが、有効な手は見出されていない。
2.3.5でlogKow用にSmall Wilson12パラメータを計算した際に、ベンジルアルコールの結果が合わないと書いた。ベンジルアルコールは、Ph-CH2-OHという構造を持つ。グループに分けると、ArCH(6), CH2(1), OH(1)になる。OHはエタノールなどと同じOHを選ぶ。しかし、ベンジルアルコールのOHは芳香属性のArOHの性質を大きく持つ。つまり、分子をバラバラにした場合に、そのグループは元接続していたグループに影響されないという独立性は無い事になる。
CCl3COOH, CF3COOHなどのハロゲン化カルボン酸も同様で、CCl3COOH, pKa=0.51, CHCl2COOH, pKa=1.26, CH2ClCOOH, pKa=2.87, CH3COOH, pKa=4.76,と酸解離定数(pKa)はカルボキシル基に何が付くかで値が大きく異なる。
化合物の沸点に対して、蒸発潜熱をプロットすると、通常の溶媒は直線に乗る。これはTroutonの通則と呼ばれる法則だ。分子が大きくなって沸点が高くなると、それに連れ蒸発潜熱も大きくなるので直線に乗る。そのような溶媒を正則溶液と呼ぶ。それに対して、水素結合を作るアルコールやカルボン酸などの溶媒は、水素結合を切る分だけ蒸発潜熱が大きくなる。
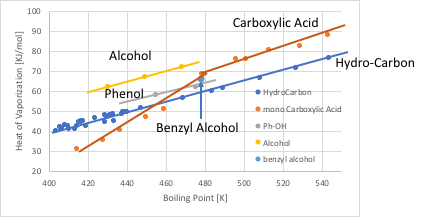
低分子のカルボン酸は2分子でダイマーを作る。そこでカルボン酸は直線では無く、屈曲点を持つ。フェノール性の水酸基は、あまり強い水素結合を作らないので、蒸発潜熱の増加量は、鎖状のアルコールより小さくなる。ベンジルアルコールは、沸点と蒸発潜熱の関係がフェノールに近いので、フェノール性のArOHと分類されるべきだ。このように、グループ間の独立性は、グループの電子吸引性の大小によって損なわれてしまう。それをプログラムに反映させるためには、蒸発潜熱を沸点で割った、Troutone Factorが非常に重要になる。また、水素結合のネットワーク構造、特にカルボン酸のダイマーに関しても、会合定数などが増える見込みは小さい。この、Troutone Factorから会合定数を見積もることも必要になるかもしれない。そして、将来的にはvkiの数え方に反映させて行く事になるだろう。本バージョンでは、グループ間の独立性については対応できていない。
Copyright pirika.com since 1999-
Mail: yamahiroXpirika.com (Xを@に置き換えてください)
メールの件名は[pirika]で始めてください。
