Hansen Solubility Parameters in Practice (HSPiP) e-Book Contents
(How to buy HSPiP)
Chapter 10
Insoluble solubility parameters (HSP for Pigment Surfaces)
A lot of the colours we see around us come
from pigments. By definition these are insoluble, so it seems to make no sense
to worry about their “solubility parameters”. Yet the HSP approach has proved immensely
valuable – giving lots of practical insight for comparatively little
work. In this chapter we’ll stay mostly in black, with the various forms of
insoluble carbon. Yet the principles apply to pigments of any hue.
It seems an admission of defeat to
introduce the concept of a pigment being “happy” in a solvent. How can such a
term apply to something as scientific as HSP? Let’s turn the question around.
As a scientist, you can shake up a sample of, say, carbon black in a solvent
and know that the pigment is happy or unhappy in that solvent. For example, a
happy carbon black will go into dispersion in a solvent with a mere shake of
the test tube. An unhappy carbon black will simply sit as a lump in the bottom
of the test tube no matter how much you attack it with high energy ultrasound.
If you use a range of solvents covering HSP space you can form a judgement of
happy/unhappy and put those data into HSPiP and calculate the HSP of the
carbon.
For those who want to be more sophisticated,
you can score the happiness in more objective ways. For example you can measure
the sedimentation rate and assign numbers on the basis that faster sedimenting
pigments have poorer solvent interaction than slower ones. However, note that
Ch.7 of the Handbook introduced the concept of RST – Relative
Sedimentation Time – to help correct for differences in sedimentation due
to density/viscosity:
RST=ts(ρp- ρs)/
η
where ts is the actual
sedimentation time, ρp and ρs are the densities of the
particles and solvent and η is the viscosity. The RST values, rather than the
raw ts values should then be used to decide between “good” and “bad”
solvents.
Either way, you will find yourself with a
plot such as the following:
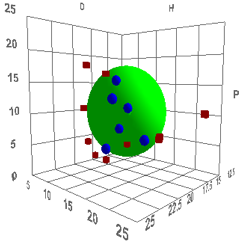
Figure 1‑1 Using file CarbonBlackLow
If you try a different type of carbon black
you find a very different result:
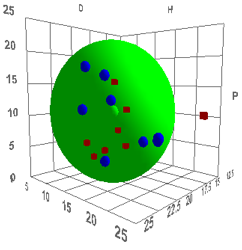
Figure 1‑2 Using file CarbonBlackHi
These two simple experiments reveal a
profound difference between two pigments both labelled “carbon black”.
|
|
δD |
δP |
δH |
R |
|
CarbonLow |
16.2 |
10.2 |
7.3 |
8.3 |
|
CarbonHi |
20.5 |
11.0 |
12.1 |
10.8 |
Table 1‑1 Comparison of parameters for CarbonLow and CarbonHi
Indeed, there are many different types of
carbon black with very different surfaces and therefore very different
abilities to interact with solvent or polymer binder. If you don’t have the
HSP, how can you rationally optimise your carbon black formulation?
If your binder and pigment have identical
HSP then you have perfect compatibility. But what do you do when you have a
coating containing pigment, binder and solvent? It seems obvious that your
solvent should also have the same HSP. But this would mean that the
binder/solvent interactions were so strong that the binder/pigment interactions
could be overwhelmed. If the binder has HSP somewhere between the solvent and
the pigment, and if the solvent is on the boundary of the binder then parts of
the binder will tend to associate strongly with the pigment, probably leaving
its solvent-compatible parts on the outside and thereby giving very good
solvent compatibility for the whole system, whilst ensuring that the binder is
nicely locked on to the pigment when the solvent evaporates.
Just pause to think on that paragraph. All
you need in order to come up with a good starting point for a practical pigment
dispersion are the HSP of pigment and binder. With help from the program you
can rapidly identify a solvent that is on the outer rim of the binder sphere,
with the pigment still further away.
Let’s try it with PMMA and the
CarbonBlackHi. Load a typical list of solvents (such as FriendlySolvents),
select PMMA in the Polymers form, make sure you’ve selected PolymerR so that
RED numbers are calculated on the basis of PMMA’s radius, and click the Solvents
button. When you look for solvents with a RED number ~1 (i.e. on the border), MIBK
looks a good fit.
|
|
δD |
δP |
δH |
R |
|
PMMA |
18.6 |
10.5 |
7.5 |
8.6 |
|
CarbonHi |
20.5 |
11.0 |
13.2 |
11.1 |
|
MIBK |
15.3 |
6.1 |
4.1 |
RED=1 |
Table 1‑2 Finding a borderline solvent
If you wanted a good place to start to
generate a good formulation using PMMA and this carbon black, then MIBK would
be a good place to start.
If you were using the CarbonBlackLow you
would, of course, choose a very different solvent.
As this is a chapter about the truly
insoluble, we introduce a pleasing digression. We came across a wonderful
YouTube video http://www.youtube.com/watch?v=jQdCRARzOv8
which shows how to determine the solubility
parameter of glass. You simply find which liquids completely wet the glass
(“Good”) and those which don’t (“Bad”) and run the Sphere correlation. We are
grateful to Dr Darren L Williams of Sam Houston State University, Texas for permission
to reproduce his data here.
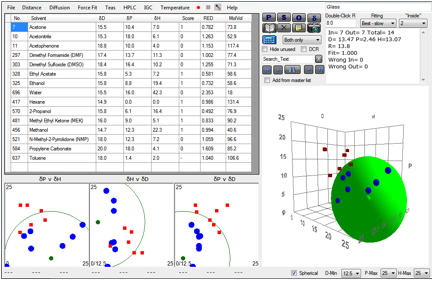
Figure 1‑3 The HSP of glass
As you can see, glass is estimated to be [13.5,
2.5, 13.1]. It will be very interesting to see if Dr Williams’ technique can be
extended to other surfaces and add insights beyond the traditional surface
energy calculations. As surface energies are often broken down into
sub-components such as Dipolar, Polar, Lewis Acid/Base it would seem an
interesting research project to see if the HSP breakdown into δD, δP, δH proves
to be fruitful in understanding surfaces. It is worth noting how unusual this
HSP set is. By using the entire Sphere Solvent Data set and putting the glass
values into the Polymer table and clicking the Solvents button, the glass sphere
is outside the entire solvent range. It will be interesting to know if this is
an artefact of the fit or a real insight into the glass surface:
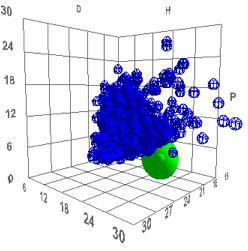
Figure
1
‑
4
The glass sphere plotted in the
context of the entire Sphere Solvent Data set, showing how unusual it is
This is our first venture out of the
“solubility” comfort zone of HSP. Let’s carry on to see another area where the
last thing you are interested in is polymer solubility.
E-Book contents | HSP User's Forum


