Hansen Solubility Parameters in Practice (HSPiP) e-Book Contents
(How to buy HSPiP)
Chapter 17, It’s your call (Rational Selection of Chemical Protective Gloves)
One approach
you could adopt is to insist on glove permeability tests for all cytotoxic
drugs on all relevant gloves. But this just isn’t practical.
An alternative
approach is to use chemical intuition: “It seems to me that glove X should have
no problem with cytotoxic chemical Y because they are chemically incompatible.”
But would you then have the courage to handle these chemicals all day with the
gloves that you have intuited should be OK? We wouldn’t.
In the absence
of specific testing, the approach we favour is a rational, numeric approach.
The numbers it produces cannot be accurate, but represent respectable estimates
of what can be expected. But we don’t need high accuracy itself. What we need
is a large margin of safety. If we estimate that a potent drug would have a
breakthrough time of 60 minutes, it doesn’t really matter if the real value is
30 minutes or 120 minutes; in neither case would we volunteer to wear those
gloves for a whole morning. But if we estimate that the breakthrough time is
360 minutes, we don’t mind if it’s really 300 or 600 minutes, the margin of safety (given
that these gloves are worn for significantly shorter periods whilst handling
the drugs) is relatively large enough for us to wear the gloves with
confidence.
Happily, we
have an excellent methodology to make this numeric estimate. We know from the
Diffusion chapter that Permeability=Surface Concentration x Diffusion
Coefficient. And we already know that a large HSP mismatch between chemical and
polymer reduces the surface concentration and therefore reduces the
permeability. A measure of the HSP mismatch is the RED number, so a large RED
means low permeability and therefore long breakthrough time.
We also know
some things about the diffusion coefficient. First, because we are handling
very potent chemicals, they will tend to be in low concentrations, so the
relevant diffusion coefficient is likely to be the D0 value, the
“intrinsic” low-concentration diffusion coefficient. There will never be enough
of the chemical to give the orders of magnitude increase in diffusion
coefficient that could give dramatic decreases in breakthrough time, nor
significant swelling of the gloves. Second, we know that the molecular shape
and size makes a big difference. There are no general rules for the effect of
molar volume but for the materials used for gloves the approximation from the
previous chapter that doubling the molar volume halves the diffusion rate would
be a conservative choice. We know, too, that linear molecules generally wiggle
their way through with more ease than branched, cyclic and/or aromatic
molecules. Once again, we can make a conservative estimate of a halving of
diffusion rate for a given molar volume if the molecule is not especially
linear. For many of the complex cytotoxic molecules we can be confident that
this is much too conservative.
We almost have
all the tools we need to estimate breakthrough times for cytotoxic molecules
through typical gloves. But we are missing a key bit of data. Although a
“large” RED undoubtedly means a “longer” breakthrough time, how do we actually
estimate the breakthrough time for a given RED number? Fortunately there are
HSP correlations for the most common types of gloves that can be used for this
purpose.
Let’s take a
specific example. From the Attacking DNA chapter we know that Cyclophosphamide
has HSP [17.5, 11.9, 12.6] and molar volume 279.
Now let’s look
at a table of glove properties. Along with the glove type and breakthrough
times is given the upper and lower molar volumes of molecules used in the
correlation. This is a useful guide for comparing to the molar volumes of the
chemical under consideration. “All” mean that there were no limitations on the
solvents.
|
Glove Type - Breakthrough |
δD |
δP |
δH |
R |
|
NR 20 MIN (58 to 178) |
17.50 |
7.30 |
6.50 |
5.10 |
|
NR 1 HR (61 to 178) |
16.60 |
9.10 |
4.40 |
10.00 |
|
NR 4 HR (58 to 178) |
19.00 |
12.60 |
3.80 |
13.30 |
|
BR 20 MIN (71 to 110) |
16.50 |
1.00 |
5.10 |
5.00 |
|
BR 1 HR (71 to 126) |
15.80 |
-2.10 |
4.00 |
8.20 |
|
BR 4 HR (All from 71) |
17.60 |
2.10 |
2.10 |
7.00 |
|
NAT 20 MIN (61 to 267) |
14.50 |
7.30 |
4.50 |
11.00 |
|
NAT 1 HR (56 to 325) |
15.60 |
3.40 |
9.10 |
14.00 |
|
NAT 4 HR (up to 325) |
19.40 |
13.20 |
7.70 |
19.00 |
|
PVC 20 MIN (61 to 267) |
16.10 |
7.10 |
5.90 |
9.30 |
|
PVC 1 HR (61 to 267) |
14.90 |
11.10 |
3.80 |
13.20 |
|
PVC 4 HR (up to 149) |
24.40 |
4.90 |
9.90 |
22.70 |
|
PVA 20 MIN (All) |
11.20 |
12.40 |
13.00 |
12.10 |
|
PVA 1 HR (All) |
15.30 |
13.20 |
13.50 |
8.80 |
|
PVA 4 HR (All) |
17.20 |
13.60 |
15.40 |
10.90 |
|
PE 20 MIN (All from 40) |
16.90 |
3.30 |
4.10 |
8.10 |
|
PE 1 HR (All from 40) |
17.10 |
3.10 |
5.20 |
8.20 |
|
PE 4 HR (All from 56) |
24.10 |
14.90 |
0.30 |
24.30 |
|
VIT 20 MIN (All) |
10.90 |
14.50 |
3.10 |
14.10 |
|
VIT 1 HR (56 to 178) |
16.50 |
8.10 |
8.30 |
6.60 |
|
VIT 4 HR (All to 178) |
13.60 |
15.40 |
8.60 |
14.40 |
|
NEO 20 MIN (75 to 178) |
17.60 |
2.50 |
5.90 |
6.20 |
|
NEO 1 HR (69 to
178) |
19.00 |
8.00 |
0.00 |
13.20 |
|
NEO 4 HR (61 to
266) |
14.60 |
13.90 |
2.30 |
15.90 |
If the
Cyclophosphamide is compared to the 20min Nitrile rubber gloves [17.5, 7.30,
6.50] and radius 5.1, the RED number is 1.5. If the comparison is for solvent breakthrough
times of 1 hour then the glove HSP values are different [16.60, 9.10, 4.40],
radius 10.0 and the RED is much reduced, to 0.88 The radius is larger because
the increased time allows less favourable molecules to diffuse through. By
extrapolation/interpolation it’s possible to say that for a RED of 1 the
breakthrough time would be 45 minutes. In other words, we have defined a radius
of Nitrile which places Cyclophosphamide in the danger
category of “soluble” and therefore we can assume, all things being
equal, that it will diffuse through in 45min if present in the sorts of
concentrations typical of the permeation cell breakthrough experiment.
But what are
those concentrations?
Again, we can
make rational estimates, but quickly add that these can be improved upon with
experimental swelling data for a given glove type.
Breakthrough time Extension
of breakthrough time Estimated Estimated
in
Permeation cell for very low concentrations
uptake,
% Dmax/D0
<20
m 4.0 25 50
20-40
m 1.9 15 10
40-60
m 1.5 10 5
60-240
m 1.1
5 2
>240
m 1.0
<3 1
For very quick
permeators, the glove material could easily hold 25% of the permeant. For very slow
permeators the glove will probably hold <3%. We can then use the typical
curves of diffusion coefficient v concentration to estimate the Dmax/D0
at those concentrations and, therefore the extension of breakthrough time for
very low concentrations.
The above
argument is sound, but the actual numbers cannot possibly be accurate. But we
don’t need extremely high accuracy. If we estimate that our intrinsic
breakthrough time is 45min and if we think (from the above table) that we
therefore have a factor of 1.5 which takes us to 70min this
really doesn’t alter our judgement very much. Whether it’s 45min or
70min we’re not going to use those gloves for a whole morning.
And when the
gloves give us >240min we don’t care if the extra safety factor is 1.1 or
1.2, we’re already in quite a good safety zone.
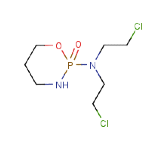
If we continued with the Cyclophosphamide example we would add
our final factor, the “shape/size” factor.
This is
clearly not a linear molecule – it’s both branched and cyclic, but then
so are some of the molecules used in the breakthrough experiments. So there’s
no room to give a significant change of D0 on those grounds. Its
molar volume is about 50% larger than any used in the breakthrough experiments
so maybe we can add a factor of 1.5 to the breakthrough time. We can finally
arrive at an estimate of 45 x 1.5 x 1.5 = 100min.
And because
it’s good practice to wear two sets of gloves (in case one gets accidentally
holed), and because breakthrough time goes as thickness squared, two gloves
take the breakthrough time to beyond 200 minutes.
For Natural
Rubber (latex) gloves the basic estimate is <20min. Even allowing for some
adjustments upwards, it seems clearly inappropriate to think of using latex
gloves, even two pairs.
For Butyl
gloves, the starting estimate is
>240min because Cyclophosphamide’s RED number at 20min is 2.8 and at 4 hours
it’s 2.0. There is no extra 1.5 for solubility because a 240min diffuser is
already at low concentrations. So we can add a 1.5 for shape/size giving use
>360min or >6 hours. That’s not bad.
Polyethylene
has such a large HSP distance from these sorts of drugs that such gloves are
obviously excellent barriers from a diffusion point of view. However, they are
easily ripped and can’t be recommended for such critical use.
As this may be
a matter of life and death, let’s repeat ourselves. The calculations on the
Cyclophosphamide and Nitrile gloves cannot possibly be highly accurate, but
they are good enough to say that Nitrile gloves are not recommended for long-term use. But for short-term
use, with a rule that two pairs of gloves should always be worn (and extra
rules if there is any chemical incident), they look OK. Similarly, the
calculations with the Butyl gloves cannot possibly be accurate, but they are
more than good enough to say that there is a considerable margin for safety for
a whole morning or for cleaning up significant spills. And that’s all we need.
Of course it’s
not up to the HSP scientist to make the final recommendations. A large number
of other considerations have to be taken into account. For example, whilst
Butyl gloves are excellent barriers, they aren’t good for delicate handling of
medicines. Latex gloves are very comfortable, but the barrier properties for
these sorts of chemicals are much too poor. Nitrile gloves are very comfortable
and are a better barrier than latex. So a hospital committee might decide, for
example, that Nitrile gloves are a good compromise choice provided that (a) two
pairs are worn, (b) the outer glove is replaced after 30min and (c) if there is
any serious incident (e.g. a spill of the chemicals) the user swaps to Butyl
gloves.
If you are
disappointed by this approach then think about how expert committees reach
their opinions on exposure limits of chemicals. They almost never have enough
good data to reach a definitive and accurate assessment of the specific risks
of a specific chemical. But usually they don’t need that accuracy. Instead they
need a defensible set of numbers to say that the risk level is in this range rather than that range. From those numerical
judgements all sorts of practical consequences can then flow. They have to make such judgements. If they
say that all chemicals are dangerous
then we can’t live a practical life. If they say “we
don’t have good enough data to form any judgement” then we live a lottery life.
If experts say “no glove is 100% guaranteed to handle all possible
cytotoxic chemicals” then their life-saving capabilities for cancer patients
will never be practically deliverable by medical professionals. If experts say “we can’t calculate any glove to high accuracies, so
just make your own judgement” we are asking the medical professionals to take
unnecessary risks, or to wait an excessively long time for experimental
results.
So we do the best
we can, with the rational tools at our disposal. If you can think of a better
method than the one described here, we’d be happy to put a note in future
editions of the eBook saying “The HSP estimator method has now been
superseded”. Till then, we think it has much to recommend it.
Some like it hot
The above
analysis may sound a little academic. Yet whilst we were writing it, Hiroshi
hit a painful problem. Unfortunately he hadn’t carried out a proper HSP risk
assessment before undertaking a task, and his hand was in pain for 3 days
afterwards.

You see, Hiroshi loves cooking with
chillies. He decided to make a large amount of chilli sauce and ground up this
large supply of chillies.
He decided
that PE gloves would be a good barrier to the capsaicin in the chillies, but quickly
discovered that they were easily damaged, so he swapped to latex gloves. After
some time handling the chillies he found that his hands were hurting from
capsaicin that had got through the gloves.
We then
decided to work out what gloves he should have used. Happily, we’d done the
work already. When we loaded the .mol file for capsaicin into the Y-MB
estimator (it’s included in the Examples folder if you want to try it yourself),
we found the estimate was similar to Cyclophosphamide and the other cytotoxic
chemicals.
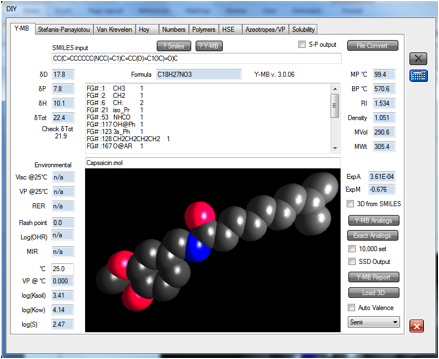
Therefore we
can recommend that the next time he has to make chilli oil, he should use two
pairs of Nitrile gloves or, if he doesn’t mind the discomfort, PE on the inside
and latex or Nitrile on the outside.
Whilst we’re
on the subject of chillies, we can address another important question. If you
accidentally eat too much hot chilli, what is the best way to remove the pain?
Because the HSP of water is too far from
capsaicin, the old favourite, cold beer, is clearly
useless. Ethanol is not a great match, but is much closer, so a sip of neat
vodka will be helpful. You often hear people say that capsaicin is “soluble in
oils”. This is only partly true. Simple oils and fats such as olive oil or lard
~ [16, 1, 5] are too far away in HSP distance to be very effective. Indeed,
Hiroshi experimented with extracting capsaicin with olive-oil
and found it made a very weak solution. However, they are better than water so
that’s one possible reason why milk and yoghurt are so often recommended as a
good way to remove the sting of chilli. It seems likely that the proteins in
milk are a reasonable HSP match with capsaicin and maybe that’s the real reason
that milk/yoghurt are recommended. However, if it’s the fats that are
important, remember not to use low-fat milk/yoghurt during your chilli crisis.
E-Book contents | HSP User's Forum


