Hansen Solubility Parameters in Practice (HSPiP) e-Book Contents
(How to buy HSPiP)
Chapter 24, Attacking DNA (HSP for DNA , Drugs, and Biological Membranes Compared)
DNA is at the centre of our lives. An
attack on our DNA is an attack on our life or on our quality of life. Such an
attack is also required of the drugs used in chemotherapy. It is therefore
rather important that we know if a chemical is likely to interact in some way
with this complex molecule, for example being cytotoxic.
A few moments thought would suggest that
HSP could have nothing to say on the subject. Cytotoxicity must be a hugely
complex activity in a complex environment.
However, when a group of well-known
cytotoxic chemicals used in chemotherapy all showed HSP values clustering
around a certain value, it seemed a good idea to check whether this was chance
or a deep insight. The gold standard of science is disconfirmation of a
hypothesis so it seemed fairly easy to hunt for cytotoxic molecules with
utterly different HSP, thereby refuting the hypothesis.
The fact that we’re writing this chapter
means that finding such a refutation has proven harder than we’d supposed!
The core data came from work designed to find gloves that were safe
for handling well-known cytotoxic drugs and is described in C.M. Hansen, Polymer science applied to biological
problems: Prediction of cytotoxic drug interactions with DNA, European
Polymer Journal 44, 2008, 2741–2748. The technique used for estimating
breakthrough times was the based on the same type of correlation as described
in the chapter on skin/glove diffusion and the following results emerged:
|
Group 1 |
δD |
δP |
δH |
V |
Ra (ave.) |
|
Fluorouracil |
18.0 |
11.7 |
11.6 |
118.3 |
1.68 |
|
Gemcitabine |
19.0 |
12.6 |
15.5 |
260.6 |
4.12 |
|
Cyclophosphamide |
17.5 |
11.9 |
12.6 |
279.1 |
2.28 |
|
Ifosfamide |
17.5 |
11.9 |
9.8 |
261.1 |
3.37 |
|
Methotrexate |
18.0 |
10.2 |
14.2 |
378.7 |
1.99 |
|
Etoposide |
20.0 |
7.5 |
12.5 |
588.5 |
4.40 |
|
Paclitaxel
(Taxol) |
18.0 |
6.6 |
9.8 |
853.9 |
4.50 |
|
Average of Group
1 |
18.3 |
10.3 |
12.3 |
- |
- |
|
Group 2 |
|
|
|
|
|
|
Cytarabine |
19.0 |
15.2 |
20.1 |
187.1 |
|
|
Carboplatin |
27.3 |
9.0 |
10.4 |
185.1 |
|
Table 1‑1 HSP properties of many cytotoxic drugs. The Ra is the distance to
the average
What is interesting is that the 4 base
segments included in DNA have the following values:
|
Segment |
δD |
δP |
δH |
V |
|
Guanine |
20.0 |
12.7 |
12.5 |
126.1 |
|
Cytosine |
19.5 |
12.1 |
9.9 |
107.8 |
|
Adenine |
20.0 |
10.2 |
13.7 |
131.5 |
|
Thymine |
19.5 |
14.2 |
12.6 |
121.7 |
|
Average |
19.75 |
12.3 |
12.2 |
- |
Table 1‑2 HSP of DNA bases
At the very least, the “coincidental”
similarity of the HSP of the bases and of the cytotoxic drugs was worth
investigating further.
For a drug to be cytotoxic it actually has
to reach the DNA. It therefore has to pass through cell walls. The chapter on
Skin has already indicated that passage through (skin) cells requires the
following HSP:
|
|
δD |
δP |
δH |
|
Skin |
17.6 |
12.5 |
11.0 |
Table 1‑3 HSP of Skin
Again, is this another coincidence?
So let’s look at another set of well-known
harmful chemicals:
|
|
δD |
δP |
δH |
V |
Ra (DNA bases) |
|
Average for
Group 1 |
18.3 |
10.3 |
12.3 |
- |
2.00 |
|
Thalidomide |
20.0 |
11.3 |
10.2 |
195.6 |
2.29 |
|
Pyrimidine |
20.5 |
9.4 |
11.3 |
78.8 |
3.39 |
|
1,2-Benzoisothiazolin
(BIT) |
20.0 |
9.4 |
9.2 |
126.0 |
4.20 |
|
Doxorubricin |
19.9 |
8.6 |
15.1 |
483.3 |
4.71 |
|
Dioxin |
20.0 |
9.2 |
7.6 |
208.2 |
5.57 |
Table 1‑4 Some well-known harmful chemicals
We now introduce the HSP distance (Ra) from
DNA bases as a predictor of cytotoxicity. By the time we reach dioxin we are at
a bigger distance and at a far less potent molecule. Doxorubricin is a potent
molecule but its distance is rather large. However, it is a complex molecule
for which the group contribution calculation may not be too accurate and
intuition suggests that the δH should be closer to 13 rather than 15.1, leading
to a distance of 3.8. It will be interesting to obtain more accurate values via
molecular dynamics or by experiment.
It’s worth attempting another challenge. So
let’s look for other cytotoxic papers in the literature. Carr J Smith’s group
at Reynolds Tobacco identified the cytotoxicity of various substituted
quinolines. The 4 most potent have the estimated HSP (using HSPiP’s
Stefanis-Panayiotou estimator) shown below. The fit with the hypothesis is
quite acceptable.
|
Substituent |
δD |
δP |
δH |
|
8-OCOCH3 |
19.9 |
7.7 |
8.6 |
|
8-NH2 |
22 |
10.4 |
12 |
|
8-OH |
20.8 |
9.8 |
14.4 |
|
8-Cl |
21.2 |
8.6 |
6.6 |
|
8-OCH2Ac |
21.9 |
7 |
4.7 |
|
Average |
21.2 |
8.7 |
9.3 |
Table 1‑5 HSP of some substituted quinolines
So far, the hypothesis is looking
reasonable. But there are plenty of other molecules with HSP in the area of
interest. How toxic are they? By entering the DNA average into the Polymer
table, selecting a Radius of 4 and clicking the Solvent button, the following
plot appears if the whole Sphere Solvent Data are loaded:
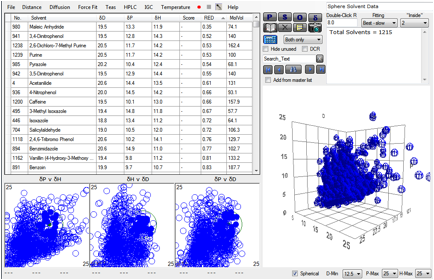
Figure 1‑1 DNA (hidden in the cloud of blue) compared to the whole solvent
range
Here we hit an immediate problem. Of those
molecules with RED < 1 both Caffeine and Vanillin stand out as chemicals we
don’t think of as cytotoxic. However, there is a large body of evidence showing
strong association of caffeine with DNA. For Vanillin there no major effect,
but there is still some doubt in the literature as to just what is going on (it
may convert to its acid form, changing its HSP).
Is this sufficient to refute the
hypothesis? Against the naïve claim that HSP match = Cytotoxicity then a
refutation is easily found. But the claim is an “HSP and…” hypothesis. We are
making the claim that the HSP match is a necessary condition for a molecule to be
able to get through to, and associate with, DNA. Necessary and sufficient
requires something more than getting through to the DNA. The obvious extra
function would be reactivity - and some anti-cancer drugs are known to be
reactive once they associate. Also, the way an associated molecule affects
binding during the replication/transcription processes will be a factor in
cytotoxicity as it may change the way that the processes are carried out.
We can use
HSP to speculate a little further. Could it be, for example, that the
well-known secondary effects of ethanol are due to its ability to “help” a
“bad” molecule to cross biological membranes? Here are two simple examples.
The
following figure shows the HSP sphere for the cytotoxic drugs. The red cubes are for ethanol, dioctyl
phthalate (DOP), and their mixture at 50/50. The 54/46 ethanol/DOP is in blue,
being defined as being just inside the sphere. This shows the distinct
possibility for synergism of given chemicals with alcohol to allow passage of
biological membranes. Once inside a cell, such chemicals can physically get in
the way of a process.
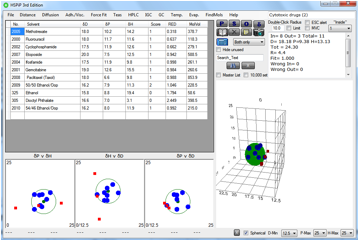
Figure 1‑2 A 54/46 Ethanol/DOP mix is shown just inside (RED=0.992) the
cytotoxic sphere
The next
figure uses the solvent optimizer with a choice of chemicals to give an
essentially perfect HSP match to the center of the cytotoxic drug HSP sphere.
Methyl paraben is already within the sphere from the start with a distance 3.26
compared to the radius 4.4. This emphasizes that mixtures of chemicals, and
perhaps especially alcohol, can give synergistic effects in biological systems.
This is clearly no proof of any effect, but deserves thought and perhaps also
experiment.
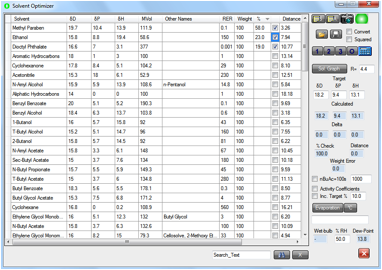
Figure 1‑3 Methyl paraben combined with DOP and ethanol produces a perfect
match
It is
obvious that we are not experts on cytotoxicity. But what we feel is that the
“HSP match is necessary” hypothesis is, at the very least, worthy of further
consideration. Because the biology world have hardly heard of HSP it’s not
surprising that they’ve not tried to take them seriously. We believe that HSP,
because of their thermodynamic grounding, are a worthy alternative to endless
QSAR correlations which provide nice numbers but lack the fundamental grounding
(and success over a wide field of research endeavours) of HSP. Perhaps this
chapter will persuade those in the biological world that it’s worth a try.
E-Book contents | HSP User's Forum


