Hansen Solubility Parameters in Practice (HSPiP) e-Book Contents
(How to buy HSPiP)
Chapter 25, HSP for Pharma and Cosmetic applications
·
Knowing the HSP of a
pharmaceutical or cosmetic ingredient allows you rationally to think through
its general bio-compatibility with DNA, skin etc.
·
Formulating ingredients for
controlled compatibility or, if required, incompatibility, is a rational
process. The evaporation modeller in the Optimizer can help you understand how
a solvent blend might change during evaporation – keeping critical components
in solution or, if you wish, ensuring that some components fall out quickly
and, for example, concentrate at the surface.
·
The analysis of skin
permeability based on Jmax instead of permeability coefficient seems to offer a
lot of insight into practical skin formulation issues – in particular
making the effects of permeation enhancers fall into a rational process of
thinking about solvency rather than the restrictive and confused terminology
such as “lipophilic v hydrophilic”.
·
The Diffusion modeller can be
used for many different issues. Permeability through skin is one example,
permeability out of microcapsules is another. The HSP distance is crucial for
understanding the solubility part of the Jmax = Solubility *
Diffusion Coefficient/Thickness equation, and the diffusion coefficient itself
can be strongly concentration dependent, another reason for knowing the HSP
distance. From knowledge of the HSP distance it is possible to predict whether
a microcapsule is likely to show Zero Order diffusion (a large distance) or
Fickian diffusion (a smaller distance). Which behaviour is desired depends on
the application, but it can be built-in rationally.
As an aid to rational chemical, excipient
or solvent use we have included a number of .hsd tables in HSPiP. The first is
a list of HSP for all the GRAS (Generally Regarded As Safe) where we could
provide a meaningful value. Here’s a snapshot from the start of the list within
GRAS.hsd:
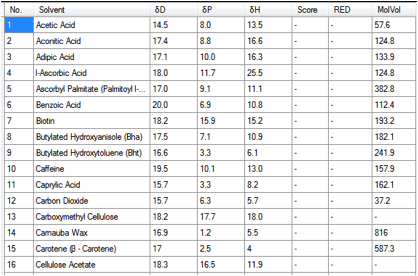
Figure 1‑1 Some GRAS chemicals
A typical use for the table would be to
find a rational substitute for a chemical that is not on the GRAS list. For
example, if your chemical were an acid with [16, 3, 8], then Caprylic Acid
would be a good starting point for substitution.
Another important list in Pharma is the
Q3C(R3) list with Type 1 and Type 2 “bad” solvents and Type 3 and Type 4 “good”
solvents. Here we’ve made it particularly easy to work out a rational
substitution of Types 1 or 2 with Types 3 or 4. We’ve provided Q3C(R3) 1 and 2.hsd
which you can load into the main form. This lets you view the key properties of
these bad solvents. For example, if you wanted to get rid of chloroform from a
formulation, you have the properties ready for the next phase:
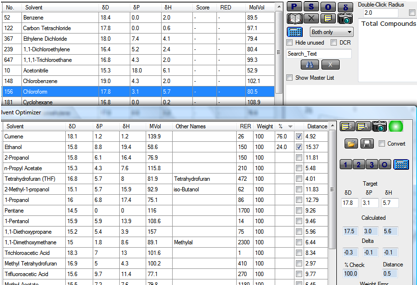
Figure 1‑2 Using Q3C(R3) to replace a “bad” solvent
By highlighting Chloroform, then going to
Solvent Optimizer into which you have loaded the Type 3 and 4 solvents as Q3C(R3)
3 and 4.sof you already have your target correctly set. Clicking on the “2”
button gives you Cumene/Ethanol as a good HSP match. Of course there is more to
matching than just the HSP. The Optimizer lets you rationally match for other
properties such as Relative Evaporation Rate etc.
We’ve done something even more. Thanks to a
generous HSPiP user we have a large list of EU cosmetic/food approved
chemicals. Via their CAS numbers Hiroshi was able to generate SMILES for a
large number of the chemicals (many others are ill-defined mixtures). He then
used Y-MB to create the Food and Cosmetics.hsd and .sof files. In searches for
alternatives in formulations, this is a most helpful. Load Food and
Cosmetics.sof into the Optimizer and you will find a rich source of
alternatives. Of course many of these aren’t solvents so you have to use
caution. But we think it’s important to give you such a large database to
explore this rich domain of “approved” chemicals. Please note, of course, that
“approval” can change and many of these chemicals come with restrictions. We
provide it as a courtesy to our users, not as an infallible guide to food and
cosmetics best practice.
Although this is a tiny chapter, we think
it’s a pointer to things to come. Solubility and permeability concepts in
Pharma and Cosmetics have, in our opinion, been dominated for too long by
one-dimensional concepts such as LogP, LogD and LogS. How much richer the
discourse becomes when scientists can discuss issues in three dimensions rather
than one!
E-Book contents | HSP User's Forum


