Hansen Solubility Parameters in Practice (HSPiP) e-Book Contents
(How to buy HSPiP)
Chapter 28, The HSP of gel formation
In the Handbook (pp271-273) a different
type of polymer gelator concept is described. An alkyd paint polymer is nicely
soluble in mineral spirits (white spirit) and, because it is not yet
cross-linked the viscosity is low – and this can lead to dripping and
spattering on application. The same alkyd (a vegetable oil modified polyester)
but with a grafted Versamid block forms a gel. The gel is easily disrupted by
stirring, brushing, or spraying – the paint is a thixotrope. The gelation
does not come from conventional polymer entanglement. Instead, the Versamid
block is insoluble in mineral spirits so it self-associates to form a loose
network, strong enough to gel but easily disrupted by mild shear forces.
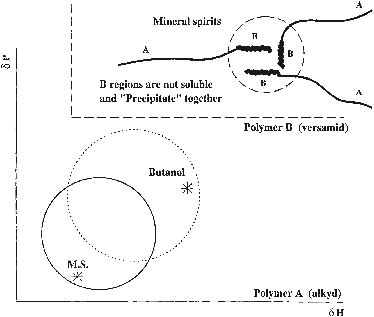
Figure 1‑1 HSP relations for establishing thixotropy in an alkyd-type paint.
The solid circle represents the solubility of the alkyd (A) and the dotted
circle that of the Versamid (B). The Versamid segments associate because they
are not soluble in the mineral spirits. Addition of n-butanol destroys the
thixotropic effect, since the solvent then becomes too good for the associating
Versamid segments and they no longer associate, being truly soluble.
To show that the thickening is not due to
classic polymer effects, if some n-butanol is added, bringing the Versamid
block into true solution, the viscosity greatly reduces and the gel effect is lost.
As another example, similar HSP concepts
have been used to carefully balance the formulation of wet-on-wet flexographic
printing inks to allow gel formation between the applying the successive
colours. This development is still in its early stages, but promises to
transform the flexographic printing industry into a greener one.
There is a third way to make gels which
uses low MWt additives. These are called LMWGs (Low Molecular Weight Gelators)
or LMOGs (Low Molecular-Mass Organic Gelators). Just 1% of an organic gelator
or 0.1% of an organic supergelator can create an impressively strong gel. A delightful
paper A. Vidyasagar, K. Handore, and K. M. Sureshan, Soft Optical Devices from Self-Healing Gels Formed by Oil and
Sugar-Based Organogelators, Angewandte Chemie, July 2011, gives you a good
idea of the effect:
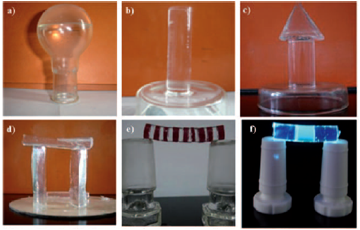
Figure 1‑2 Gels formed in pump oil or silicone oil with ~0.3% of organic
gelators. Typical dimensions in the images are 2-5cm. For explanations of
shapes and colours, please read the original paper.
They are prepared by heating the gelator in
the solvent then cooling to the gel phase. Such gels can be used in many
applications such as optics, printing, cosmetics, coating etc.
The way they work is that the gelators have
a tendency to self-aggregate into long chains and networks – forming a
virtual polymer.
So how does one choose the right gelator
for their particular system? It seems beyond the state of the art to be able to
predict ab initio that molecule A
will gelate system B. Instead one has to look in the literature for molecules
that happen to gelate systems based on B. If you are lucky you will find a
molecule that is cheap, safe, effective and perfect for B. But suppose, for
example, that you want to gelate a cosmetic formulation based on isopropyl
myristate (IPM) and you can’t find any literature references to gelators for
IPM. How do you then choose a gelator? You look for solvents that have been
successfully gelled by a given gelator and that are also “close” IPM, but how
“close” does it have to be?
The key problem is obvious. By definition
the gelators like to self-associate. So a poor solvent for the gelator will not
break up the self-association and the gelator will be simply insoluble. At the
other extreme, really good solvents will be so good that the gelator doesn’t
self-associate so you simply have a solution.
The problem is solved, therefore, by
finding areas of marginal solubility – not too little and not too much.
And how would you find this? HSP seem to be the natural way to approach the
problem.
We are most grateful to Matthieu Raynal and
Laurent Bouteiller for giving us permission to use their wonderful paper Organogel formation rationalized by Hansen
solubility parameters, Chem. Commun., 2011, 47, 8271–8273 to show you
how it’s done. The datasets are taken, with their kind permission, from the
Supplementary Data to their paper.
They did a simple HSP scoring of 0 and 1
for insoluble/soluble and, separately, for no-gel/gel. You therefore get two
HSP values, one is the classic “solubility” and the other is the “gelator” value.
Knowing these values for the range of 8
gelators that span a wide range of structures it then becomes possible to find
which one has a gelator sphere suited, for, say, IPM.
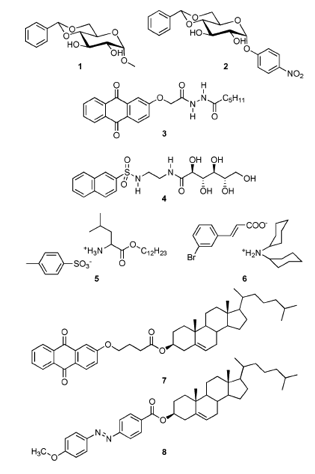
Figure 1‑3 The 8 gelators used in the study. We will focus on 1 & 2 and 7
as they represent opposite ends of the gelator spectrum - (1) involves
H-bonding, (7) involves π-stacking.
For these gelators, the best fits were
obtained with GA Classic mode. Here are the results:
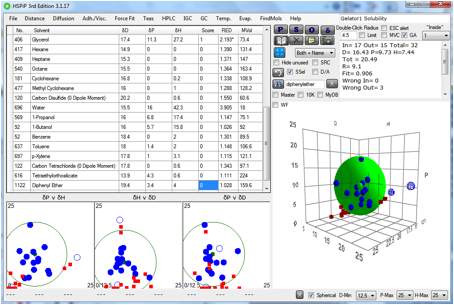
Figure 1‑4 The solubility sphere for Gelator 1
The gel sphere, not surprisingly, is much
smaller and in a very different region:
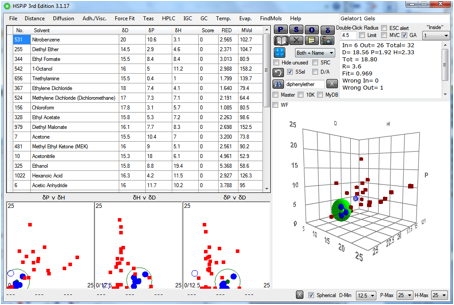
Figure 1‑5 The gel sphere for Gelator 1
The HSP distance from this gelator sphere
to IPM ~[16, 4, 4] is greater than the radius of 3.6 so this gelator will
probably not be useful.
For Gelator 7, which relies on aromatic
pairing for gelation rather than –OH bonds, the spheres are very
different.
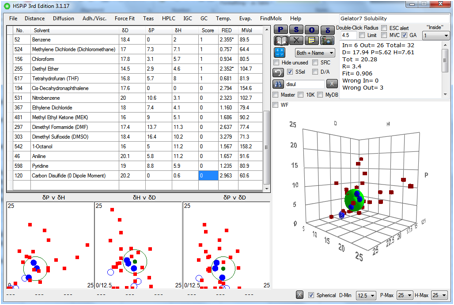
Figure 1‑6 The solubility sphere for Gelator 7
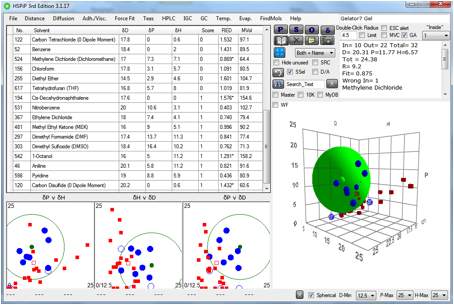
Figure 1‑7 The gel sphere for
Gelator 7
Again, this is unlikely to be a gelator for
IPM
Finally, when we try Gelator 2 we get a
solubility sphere (not shown) at [18.3, 13.8,10.1] and a gel sphere which is
close enough to IPM that it might well be a reasonable gelator for it:
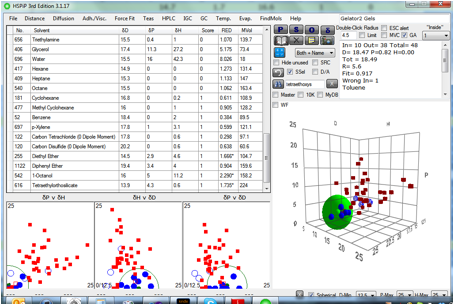
Figure 1‑8 The gel sphere for Gelator 2
As the authors point out in their paper,
not all the fits are perfect, and some of the datasets need careful
interpretation as gelation behaviour is complex with time-dependent effects
that can make what looks like a gel become a precipitate after a time.
Another suggestion from the authors is to
create double-sphere fits. The reason is that although two different solvents
can both be gelators, the type of gel (the network fibre structure) can be
different.
The double-sphere option in the GA mode is
ideal for this. As you can see in the above fit for Gelator 2, some of the gel
solvents are outside the fitted sphere. Here are the same data fitted with the
double-sphere option.
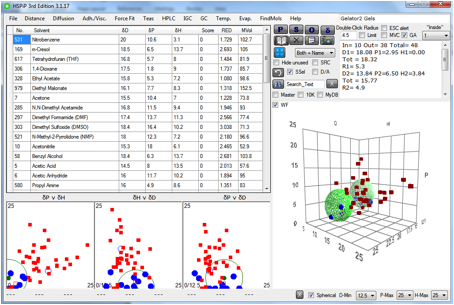
Figure 1‑9 A double-sphere “fit” to Gelator 2. The value for the second sphere
is unconvincing.
In this example it is clear that the fitted
values are rather unconvincing – a δD of 13.8 is unlikely. Perhaps it
would be better to do a sub-analysis of the different types of gel (if they
exist for this particular molecule) and fit them separately.
The point of this brief chapter is not to
draw definitive conclusions about using HSPiP with gelator data but to show
that this sort of analysis may well be highly fruitful for further exploration.
E-Book contents | HSP User's Forum


