Hansen Solubility Parameters in Practice (HSPiP) e-Book Contents
(How to buy HSPiP)
Chapter 29, Going nano (HSP Characterizations of
Nanoparticles)
It’s obligatory to have a nano chapter
because nano is new and exciting. In fact the truth is that nano is old and HSP
have been solving nano issues for decades. We know this because the chapter on
“insoluble” HSP is all about carbon black and carbon black has been nano ever
since it first appeared as smoke.
But this chapter is a reminder that those
working at the cutting edge of science could do well to remember older, simpler
principles. We start with that great symbol of nano-modernity, the carbon
nanotube, CNT.
Note.
The following section was written before the work of Detriche et al (see below)
was published. It is interesting to compare our predictions with those of the
Detriche paper.
Although there are some papers that
specifically invoke HSP for understanding the best solvents for dispersing CNT,
we have found the data to be rather unsatisfactory. First, these papers don’t
have a sufficiently full range of solvents to define the sphere in 3D. Second,
we think that the data for good dispersion is skewed by high density or high
viscosity liquids. Because the test for a “good” solvent is whether a
dispersion remains stable over time, a high density or viscosity can give a
misleading result. Ch.7 of the Handbook introduced the concept of RST – Relative
Sedimentation Time – to help correct for differences in sedimentation due
to density/viscosity:
RST=ts(ρp- ρs)/
η
where ts is the actual
sedimentation time, ρp and ρs are the densities of the
particles and solvent and η is the viscosity. The RST values should then be
used to decide between “good” and “bad” solvents.
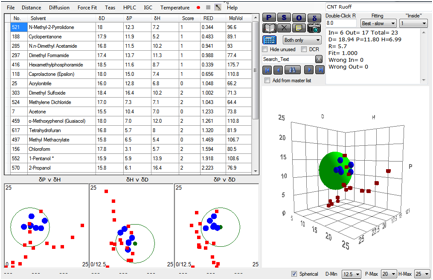
Happily a paper K.D. Ausman, R. Piner, O. Lourie, and R.S.
Ruoff, Organic Solvent Dispersions of
Single-Walled Carbon Nanotubes: Toward Solutions of Pristine Nanotubes, J.
Phys. Chem. B. 104, (38), 8911-8915, 2000 gives a range of good solvents
which, when combined with those known to be bad gives the following excellent
fit:
Figure 1‑1 Fit of Ruoff’s CNT data
The values aren’t too far from the less
reliable fit which gave a value in the [18, 10, 9] region. The Solvent Optimizer
readily informs us that DMF/Xylene (69/31) or Caprolactone/ Dipropylene Glycol
Mono n-Butyl Ether (67/33) would be mixtures superior to any of the individual solvents
used in the above experiment.
The paper by Detriche et al Application of the Hansen Solubility
Parameters Theory to Carbon Nanotubes, J. Nanoscience and Nanotechnology, 8,
1-11, 2008 is an interesting vindication of the comments above. Although
the paper covers many different types of CNTs, the data below are specific to
Single Wall Nanotubes, SWNT.
First, they confirmed that without thorough
centrifugation of dispersed samples results are highly unreliable as viscosity
and density can skew the apparent solubility/dispersability of CNT. Their paper
casts some doubt on some of the “good” solvents used in the fit above, which
may partially explain differences between the two studies in the calculated
values for CNTs.
Second, they showed that mixtures of two good
solvents can give better solubility/dispersability. So a 50/50 mixture of
o-dichlorobenzene/benzaldehyde gave a higher (8x) solubility than either alone.
|
|
δD |
δP |
δH |
Ra |
|
SWNT |
19.4 |
6.0 |
4.5 |
Radius=3 |
|
o-Dichlorobenzene |
19.2 |
6.3 |
3.3 |
1.30 |
|
Benzaldehyde |
19.4 |
7.4 |
5.3 |
1.61 |
|
50/50 mix |
19.3 |
6.9 |
4.3 |
0.9 |
Third, and an excellent vindication of HSP
principles, is the fact that a 75/25 mixture of two non-solvents gave a
reasonable solubility
|
|
δD |
δP |
δH |
Ra |
|
SWNT |
19.4 |
6.0 |
4.5 |
Radius=3 |
|
Diethyl
phthalate |
17.6 |
9.6 |
4.5 |
5.1 |
|
Methyl
naphthalene |
20.6 |
0.8 |
4.7 |
5.7 |
|
75/25 mix |
19.3 |
6.9 |
4.3 |
3.1 |
The authors had also noted a flaw in their
initial study with 16 solvents. The chosen solvents did not give a sufficiently
good bracketing of HSP space so the Sphere calculation could not produce a
reliable fit. They could have used more solvents. But instead they used a large
number of solvent mixes which, of course, achieves the
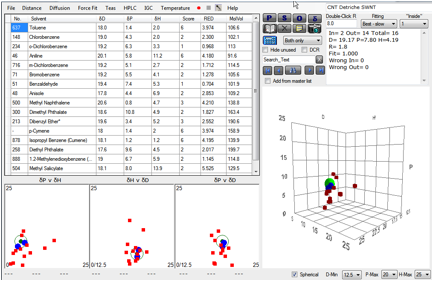
same thing. A plot of their 2 best single
solvents gives the following:
Figure 1‑2 Fit of Detriche CNT data
If the “2” solvents are used then the
result is meaningless sphere. But by using the mixture range they were able to
bracket the CNTs and obtain the value shown in the table above.
Fifth, they recognised a further HSP
principle – that larger polymers have smaller radii. They were able to
fractionate CNTs using this principle – the average CNT size in a “good”
solvent was considerably smaller than the size in a “very good” solvent.
Sixth, though it hardly needs pointing out,
Hildebrand parameters proved a poor way of predicting solubility behaviour.
Surfactants are also a popular method for
obtaining good dispersions of CNT in water. So far we have not been able to
find a good correlation between the HSP of the surfactants and their dispersion
capabilities for the CNT. The standard hydrophile/hydrophobe chains in these
surfactants simply don’t seem to have the capability of producing HSP with the
required high δP and δH.
Two papers from the Coleman group
explicitly use HSP to further push the boundaries of CNT and graphene
solubilities. Shane D. Bergin, Zhenyu Sun, David Rickard, Philip V. Streich,
James P. Hamilton, and Jonathan N. Coleman, Multicomponent
Solubility Parameters for Single-Walled Carbon Nanotube Solvent Mixtures,
ACNano, 3, 2009, 2340-2350 and Yenny Hernandez, Mustafa Lotya, David Rickard,
Shane D. Bergin, and Jonathan N. Coleman, Measurement
of Multicomponent Solubility Parameters for Graphene Facilitates Solvent
Discovery, Langmuir, DOI: 10.1021/la903188a. The HSP analysis (the authors
used HSPiP during their research) is not perfect and doesn’t explain
everything, but such huge “molecules” really are pushing the boundaries of HSP.
Nonetheless, it’s clear that HSP do a quite impressive job in helping
understand these complex phenomena.
Similarly, recent work from the Delhalle
group throw more insight into CNT surfaces, especially the fact that many
“functionalised” CNT are very poor mixtures of undefined quality. See Detriche,
S., Nagy, J.B., Mekhalif, Z., Delhalle, J, Surface
State of Carbon Nanotubes and Hansen Solubility Parameters, Journal of
Nanoscience and Nanotechnology, 9, 2009 , 6015-6025.
C60
We can’t miss the chance to discuss C60.
There have been numerous attempts to understand the slightly odd solution
behaviour of C60. Not many other chemicals are best dissolved in chloro- or
phenyl-naphthalene at room temperature. Many efforts using QSAR techniques
sifted from 1000’s of possible “molecular” properties have produced
correlations with R² from 0.6 (poor) to 0.9 (not bad), but with little
apparent insight into what is really going on. Hansen and Smith used basic
Sphere techniques in their paper C.M. Hansen, A.L. Smith, Using Hansen solubility parameters to correlate solubility of C60
fullerene in organic solvents and in polymers, Carbon, 42 (2004) 1591–1597
to find HSP of [19.7, 2.9, 2.7] and thereby to make predictions of 55 solvents
that might do a reasonable job and then went on to show which polymers
(polystyrene is a good example) would have good compatibility with C60.
We’ve taken advantage of a rather expanded
data set to re-do the correlation. Here is the Sphere with 107 solvents instead
of the original 87. The definition of good “1” solvents is
Log(MoleFraction)>-3.0.
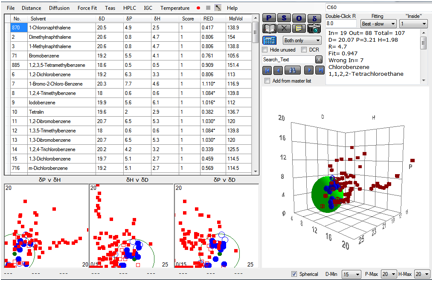
Figure 1‑3 Fit of Hansen C60 data
The result is [20, 3.2, 2.0] which is close
to the original fit. If the definition of “good” solvents is changed to include
all the “2” solvents, defined as Log(MoleFraction)>-4 the value changes to [20.5,
3.8, 1.6], only a modest change.
Because there is such a tradition of doing
least-squares fits of the Log(MoleFraction) data, we did the same, using the
formula:
Log(MoleFraction) = K * Sqrt(4*(δDc60-δDs)2
+ (δPc60-δPs)2 + (δHc60-δHs)2)
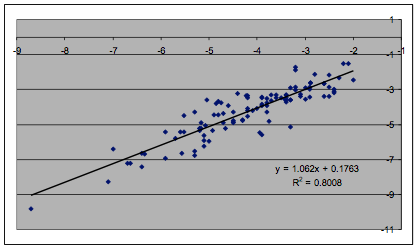
Figure 1‑4 Least squares fit of Hansen C60 data
The R² is a respectable 0.8 when the
C60 HSP are set to [22.5, 0.6, 2.9], remarkably close to the Sphere fit.
Of course there’s more to solubility of a
molecule like C60 than enthalpy. There must surely be some entropic effects
and, presumably, some specific inter-molecular effects. But straightforward HSP
do a remarkably good job at covering this large range of solubilities and,
importantly, provide practical predictions on solvents and polymer
compatibility that the working scientist can combine with intuition and
experience to help develop processes for C60. The fit is close to the best
published fits via QSAR without the need for those 1000+ factors available to
practitioners of that art. The bottom line is that C60 is in an awkward
position in solubility space – not many liquids have δD values in the 20+
range and even less combine that with low δP and δH values. Chemicals with such
high δD values are (usually) solids. It’s the δD which makes it so hard to find
convenient solvents for processing C60, it’s as simple as that.
And for those who wish to test out a
prediction. From published data of cohesive energy density, sulphur comes out
with a value in this high δD range so it’s probably a good solvent if you wish to
try it.
Graphene
too
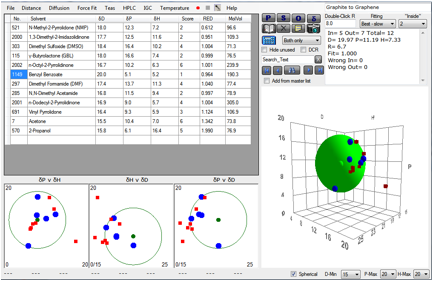
The amazing properties of graphene hold great promise for many
applications. Geim’s initial method for producing it using adhesive tape is
breathtakingly simple and inspired but not adequate for mass production. The
paper by Coleman and his large team, High-yield
production of graphene by liquid-phase exfoliation of graphite, Nature
Nanotechnology, 3, 563-568, 2008, shows that graphene is soluble in a solvent
as simple as Benzyl Benzoate and therefore potentially graphene coatings can be
produced direct from solvent. We’ve plotted their data (with their permission) in
HSP terms and obtained the following value for the HSP of graphene:
Figure 1‑5 Fit of Coleman’s Graphene data
When [20, 11.2, 7.3] is put into Solvent
Optimizer, a near-perfect match is obtained by a 60:40 blend of Caprolactone
and Benzyl Benzoate. It will be interesting to know if this blend is actually
better than Benzyl Benzoate alone.
Nano-clays
Clays are remarkably cheap and, with a bit
of exfoliation, are remarkably nano. A lot of people have therefore spent a lot
of time trying to make polymer/clay nanocomposites. With hindsight it is clear
that a lot of this work has been wasted because, first, exfoliating the clays
is not easy and, second, it is not obvious which organic groups would be most
compatible with a given polymer.
The best-known method for aiding
exfoliation is to create an organoclay via ion exchange to remove the sodium
ions between the plates and replace them with a quaternary ammonium salt,
typically containing a mixture of methyl, benzyl, hydroxyethyl and tallow
groups. If this is done well then the resulting clay contains neither excess
sodium nor excess quaternary salt. But doing things well makes the clays more
expensive. But by using impure versions, interpreting the data is very
difficult.
Assuming the organoclay is of high quality,
what is the best one to use for any given application? Usually the ideal is
total exfoliation of the clay within the polymer. But many users are happy if
they have lots of “tactoids” (nano clusters of clay particles) which are, at
the very least, better than mechanically dispersed clay microparticles.
At this stage it would be good to show that
HSP can come to the rescue.
Unfortunately, the data don’t allow the
production of good HSP. The file Clay4 fits the data from D.L. Ho and C.J.
Glinka, Effects of Solvent Solubility
Parameters on Organoclay Dispersions, Chem. Mater. 2003, 15, 1309-1312. The
clay is dimethyl-ditallow montmorillonite (Cloisite 15A) and gives an HSP set
of [18.2, 3.8, 1.7]. Unfortunately, attempts to fit (nominally) the same clay
from the data of D. Burgentzlé et al, Solvent-based nanocomposite coatings I. Dispersion of organophilic montmorillonite
in organic solvents, Journal of Colloid and Interface Science 278 (2004)
26–39, shown in Clay2, gives the impossible values of [16.8, -4.7, -3.3].
The problem is compounded by the fact that the solvent data contains its own
uncertainties. Does one class as “good” solvents those that swell the clay or
those that cause a big increase in the interlayer spacing? Furthermore, one of
the really good solvents in the first paper, chloroform, is a bad solvent in
the second.
From the second paper, the dimethyl-benzyl-tallow
(Clay1 – Cloisite 10A) gives [20.4, 6.6, 5.9]
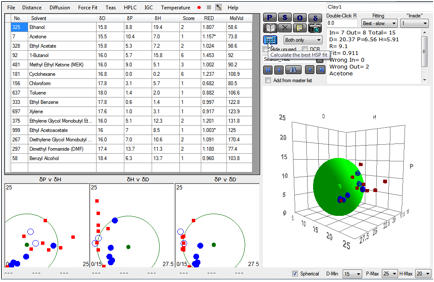
Figure 1‑6 Clay 1 fit
and the methyl-di(hydroxyethyl)-tallow
(Clay3 – Cloisite 30B) gives [15.8, 15.2, 11.0]. Adding n-alcohol data
from another paper on the 30B gives [16.7, 10.4, 10.2], though there is a
contradiction with the data point for ethanol.
Because organoclay nanocomposites look to
be of such great importance it would seem a good idea to re-test the solvent
swelling data on a group of well-defined clays, using a larger range of
solvents across HSP space to gain a better set of values or, conversely, to
show that for some reason the HSP approach is not appropriate.
Nevertheless, when one of us (Abbott) tried
applying the HSP data to a group of papers on organoclays in poly(lactic acid)
[18.6, 9.9, 6.0], it became obvious that the popular 30B was less likely to be
a good match than the 10A, whilst the also much-used 15A was likely to be
unsatisfactory. The revised data on 30B reduced the degree of mismatch with the
poly(lactic acid) – re-emphasising the need for a definitive data set on
these clays.
Recent work by a team led by Dr Andrew
Burgess (then in ICI, now in Akzo Nobel) gives some visual elements to this
story. We are grateful to Dr Burgess for permission to use their material here.
They used a set of Cloisite clays and attempted to disperse them in a range of
solvents. Typical results for four solvents are shown:
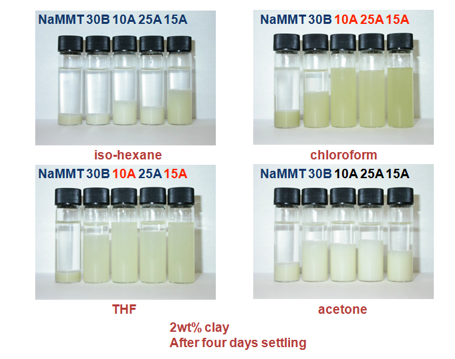
Figure 1‑7 Some of the data for Cloisite clay dispersions from A. Burgess, D.
Kint, F. Salhi, G. Seeley, M. Gio-Batta and S. Rogers, reproduced with
permission.
For example, it’s clear that chloroform is
good at dispersing/swelling 10A, 25A and 15A, whilst THF is only good for 10A
and 15A. i-Hexane and acetone don’t do a good job with any of the clays. From
their full dataset they tried an analysis using Hildebrand solubility
parameters. The results were unconvincing. The same data put into HSPiP allowed
a more insightful analysis. Here, for example, are the data for the Cloisite
10A:
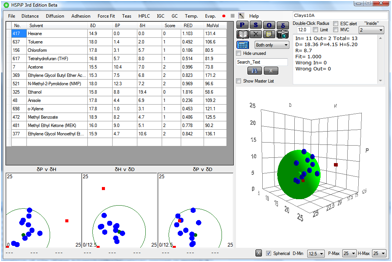
Figure 1‑8 A fit for the Cloisite 10A results from Burgess et al.
As the files are provided with HSPiP you can
judge for yourself how good or bad the fits are. A larger range of solvents
would, as always, provide greater certainty. But a retrospective analysis of
the Burgess’ team’s attempts to combine the clays with various common acrylates
showed that the HSP were a good indication of the relative ease or difficulty
of making stable clay dispersions.
Quantum
dots
When a particle of something as ordinary as
CdTe becomes smaller than ~10nm then its electronic properties are governed by
the wave function that can fit inside the dot rather than the properties of the
material itself. So CdTe can become green, red or blue depending on the
particle size. There are numerous applications for such quantum dots. But
because small particles have large relative surface areas they tend to clump
together, losing their quantum-dot nature and/or their ability to be dispersed
in the medium of choice.
Because there are so many different quantum
dots, stabilised by a large variety of different methods, there seems to be no
general theme emerging for which HSP give an over-arching insight. However, one
data set kindly provided to us by Michael Schreuder working in Professor
Rosenthal’s group at Vanderbilt University shows a mixture of the expected and
the unexpected. CdSe nanocrystals were stabilised with a shell of a substituted
phosphonic acid. Here is a typical example of a fit with 43 solvents for the
Butylphosphonic acid system:
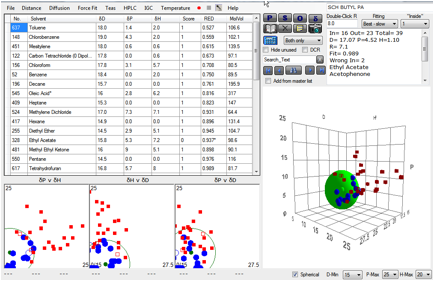
Figure 1‑9 The fit to a Quantum Dot
The calculated values [17.0, 4.5, 1.1, 7.1]
seem reasonable for a somewhat polar butyl chain. The problem is that when one
goes to the phenyl phosphonate, the values are remarkably similar. The fit of
[16, 4.7, 2, 5.3] has a disturbingly low δD value. The fit for the
Octylphosphonic acid version [17, 3.7, 1.5, 6.8] does not show the expected
decrease in δP and δH for the longer alkyl chain. And, surprisingly, the
2-Carboxyethylphosphonic acid fit [16.4, 4.8, 3.2, 4.7] shows no evidence for the
expected higher δP and δH. Even worse, some of the fits (not included, for
reasons we’ll describe in a moment) were very poor quality.
But maybe we are jumping too quickly to
conclusions. We’re assuming that the CdSe surface is entirely covered by a
shell of substituted phosphonic acids, with the chains sticking out into the
solvent, so the HSP should be that of the chains. But what if some of the CdSe,
or the phosphonate group is accessible to the solvent – how much would
that contribute to the HSP? Conversely, what would happen if there were still
an excess of the phosphonate – that would give strange results. The
investigators checked out this last possibility. The samples that gave poor
results were checked using Rutherford Backscattering and were found to contain
excess phosphonate. At the time of writing, the reason for the relatively
uniform HSP for the range of phosphonates has not been found, but it is
satisfying to note that when some poor fits suggested either that the HSP
approach was wrong or that the samples themselves had issues, it was the latter
that was found. This does not prove that HSP are right, but once again it shows
that they can be deeply insightful even down to the level of quantum dots.
E-Book contents | HSP User's Forum


