Hansen Solubility Parameters in Practice (HSPiP) e-Book Contents
(How to buy HSPiP)
Chapter 6, Safer, Faster, Cheaper (Optimizing Solvent
Formulations)
With the Optimizer you can quickly home in
on the HSP of your target. Let’s assume, as in Coming Clean, that we want to
dissolve a polymer in an ink. If we know that the polymer has HSP of [18.6,
10.1, 7.8] then we can use HSP tables to find the best match. From a large list
you can find that N-Acetyl Piperidine and Hexamethylphosphoramide are excellent
matches, but you are not likely to want to use either of those!
Out of your 19 solvents, the RED number
shows you that N-Methyl-2-Pyrrolidone is not a bad match, but it’s expensive,
slow to evaporate and has some health & safety issues.
It looks as though we’re stuck. But
remember that a solvent blend with the same parameters as the polymer is
thermodynamically identical to a pure solvent. So if we can’t find the perfect
single solvent, let’s find a blend.
If you’ve ever tried doing this manually
from a list of solvent HSP you will know that it’s a bit of a slog. So let’s
get the computer to do it.
The Optimizer comes with a list of
“friendly” solvents – ones that you might find in general use and which
aren’t too toxic or expensive. Everyone’s definition of “friendly” differs so
you should feel free to modify the list for your own purposes.
When you enter the Target HSP (in this case
[18.6, 10.1, 7.8]) you can then select one or more solvents and their % and
click the Calculate button to compute the HSP of the blend – which is
simply the weighted sum of the individual components. The Distance is also
automatically calculated – the smaller it is, the better.
This is helpful for scouting purposes but
it is hard to find an optimum this way.
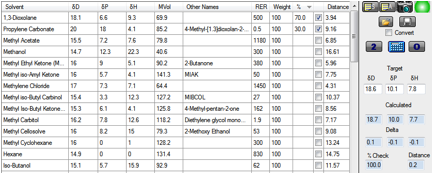
A quick short-cut is to click the 2 button which does an
exhaustive search of all possible combinations of 2 solvents to find the
closest match (smallest Distance). When we do this, a blend of 1,3-Dioxolane
and Propylene Carbonate is chosen.
Figure 1‑1 Solvent Optimizer trying to match the polymer’s [18.6, 10.1, 7.8]
This will undoubtedly be a fast dissolving blend.
Both molecules have a small Molar Volume (MVol) and small means fast diffusing
(kinetics) and large entropy change (thermodynamics) for good dissolution.
So we have Faster. But what about Safer?
The flash point of 1,3-Dioxolane is rather low so you might not like to include
it. If you go into the main program and load the full set of solvents you can
do the “Double Click” trick on 1,3-Dioxolane to find that Tetrahydofurfuryl
alcohol is not too far away from it. That is certainly not too volatile. So
let’s deselect the Dioxolane and select the alcohol. What % should be used? The
simplest way to find out is to click the Optimize button. It turns out that a
75:25 mixture is optimal. The Distance is a bit larger, but it should still be
OK. The MVol is also OK. So now we have Faster and Safer. Unfortunately,
Tetrahydofurfuryl alcohol is rather expensive. So we have to work a bit harder
to get Cheaper. By exploiting some Advanced Optimization tricks within the
Optimizer it’s possible to find that a combination of Dipropylene Glycol (DPG)
and Aromatic Hydrocarbons is not a bad match for Tetrahydofurfuryl alcohol. So
now we click the Optimizer button once more and find that we have got a good
blend of Faster, Safer and Cheaper with these 3 solvents:
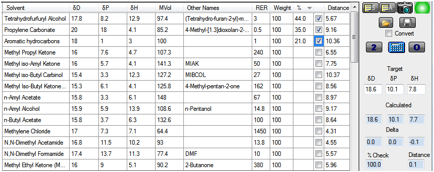
Figure 1‑2 A blend optimized by clicking the O button
Of course there are always trade-offs. This
blend has the relatively high MVol of the DPG so Faster has been compromised
– but if this is a priority then you can carry on searching in a rational
manner to replace the DPG with something smaller.
For those who wish to avoid Aromatic
hydrocarbons it’s possible to find blends of 4 or 5 components that do a good
job. We leave that as an exercise for the reader.
Let’s remind ourselves what we’ve done in
such a short time. With 16 simple tests of whether a solvent dissolved or
didn’t dissolve our ink we found the HSP of the ink. We did 3 more tests just
to refine the value. Then after about 30 minutes on the computer we found a 38:33:29
mix of Propylene Carbonate, Aromatic hydrocarbons and DPG as a Faster, Safer,
Cheaper blend. Can you imagine how long it would have taken you to find such a
blend without HSP?
It’s no coincidence that solvents with this
sort of mixture of aromatics, propylene glycols and high P solvent are widely
used in the “safer solvents” industry. Indeed, one of us (Hansen) helped found
a Danish “safer solvents” business on the basis of patents derived from the
insights of HSP.
When
in doubt go higher
If you had a choice of two solvents, the
same distance from the target, and one is of low δTot and the other is of high
δTot, which one should you choose? Hansen’s view is that higher is better. Why?
The analogy is with the Kauri Butanol number. A “poor” solvent causes the kauri
to crash out after relatively little dilution, a “good” solvent is tolerated to
a much greater extent. Because (by definition) butanol is used in the test,
high δTot solvents are likely to be more compatible with the butanol and
therefore limit the crashing out of the kauri. Armed with HSPiP one could
probably find plenty of exceptions to this rule of thumb, but to the extent
that the Kauri Butanol number is of any value (and that’s debatable) the
“higher is better” rule is a reasonable guide.
Squared
mixing algorithm
For the past 40 years, the simple weighted
volume average discussed above has proved to be an acceptable way to formulate.
However, an argument can be made that a “squared mixing algorithm” should be
used. For example, if solvent 1 and solvent 2 are present in volume fractions
of x and y then each δ of their mixture would be:
δmix= sqrt(xδ12
+ yδ22)
Because this is cumbersome to apply it’s
seldom been used. For the 3rd Edition we have added the option. We
will be very interested in user feedback on whether it is, in fact, superior to
the linear algorithm. For modest differences in δ the two algorithms give
results within the usual margin of error; the algorithms only diverge
significantly when there are very large differences in δ.
In the next chapter we show how HSP can make
the incompatible, compatible.
E-Book contents | HSP User's Forum


