Hansen Solubility Parameters in Practice (HSPiP) e-Book Contents
(How to buy HSPiP)
Chapter 9, Shades of Black (Designed Partial
Compatibility - Bitumen)
This
chapter is based on the work by Per Redelius of Nynas Bitumen which appeared in
Chapter 9 of the second edition of the Handbook. His data are reproduced by
permission.
It is very easy to take bitumen (usually
called asphalt in the US) for granted. It’s just black sticky stuff that comes
from oil.
That’s true till your life depends on it.
Road safety depends strongly on the qualities of the road surface which in turn
depends on the subtle details of the bitumen used to make it. More
sophisticated road surfaces use bitumen that has been modified with polymers.
It is important to know the compatibility between the bitumen and the polymer.
Bitumen is produced by distillation from
crude oils and its properties can vary widely depending on the crude oil used.
Unfortunately it is exceedingly difficult to analyse. And because it is so
black and viscous, it is hard to know whether, for example, you have a solution
or a dispersion when you add a polymer to the bitumen. And of course whether it
is dissolved or dispersed makes a big difference to the ultimate properties of
the bitumen and the road surface.
We know that a thermodynamic definition of
“compatibility” is provided by HSP. So if we know the spheres of the bitumen
and the polymer we can calculate how much they overlap. If the overlap is
strong we can be confident of compatibility. If the overlap is small then
compatibility will be unlikely.
First we need to characterise our bitumen.
From the solubility in 42 solvents we can calculate the properties:
Well, actually, it’s not quite that simple.
It’s very hard to know if you’ve got a perfect solution or if there are some
bits or if it’s merely swollen or... A Yes/No solubility test is rather hard to
apply. So you can score the solubility from 1-6 where 1 means that there’s not
the slightest residue if you filter the solution to 6 which means there’s not a
hint of colour in the liquid. You then need to define what you mean by “good”
or, in the language of HSPiP “Inside” the Sphere. If you’re really careful,
you’ll define “Inside” as “1”. Or you might be interested in “good enough to be
inside” so define “good enough” as “3”.
This brings in the crucial question of
subjectivity in HSP calculations. How can I tell the difference between a score
of 2 and a score of 3? How worried should I be about a mis-scoring?
The good news is that the software lets you
try out the effects of allowing different scores to be classed as “Inside”.
With this particular bitumen, the variation in parameters between 1 & 2 is
not all that great. When you admit 3’s as “Inside” then the radius (not
surprisingly) increases.
|
Inside score |
δD |
δP |
δH |
R |
|
1 |
18.4 |
5.8 |
2.9 |
6.2 |
|
2 |
17.9 |
5.1 |
3.1 |
5.8 |
|
3 |
18.2 |
3.8 |
4.6 |
9 |
Table 1‑1 How the Sphere parameters depend on the definition of “Inside”
The plot below (based on a generic bitumen)
uses a 0-15 scale for P and H to provide a more detailed view for this and
subsequent plots.
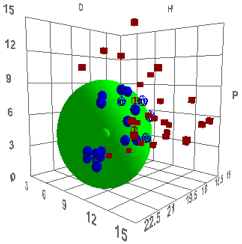
Figure 1‑1 Using file Bitumen
So now we can start looking at different
polymers. If we try polyether sulfone we find that the spheres are highly
distinct – so it is unlikely that the polymer will be anything other than
a dispersed solid when mixed with the bitumen. To get this plot, open the
Polymer form and enter the Bitumen data as the last item. Select Bitumen and
also select Polyethersulfone. Then click Solvents and the two polymers and the
solvents are plotted for you.
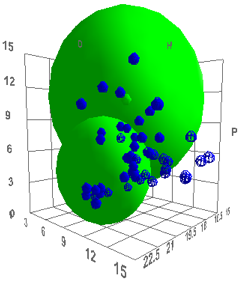
Figure 1‑2 Comparison of Bitumen and Polyethersulfone
A plot comparing bitumen with
Styrene-Butadiene block co-polymer (SBS) is more interesting because SBS is the
key polymer used with bitumen in road construction.
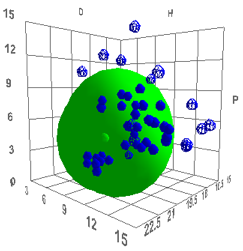
Figure 1‑3 Bitumen and an SBS polymer #271
It shows significant overlap but a significant
part of the SBS is outside the bitumen. Given that SBS has two very distinct
polymeric components it is interesting to note that if you compare polystyrene
with polybutadiene you see that they are not very mutually compatible –
which is why SBS is an “interesting” polymer. And if you calculate the overlaps
between bitumen, polystyrene and polybutadiene you find that they have similar
degrees of overlap.
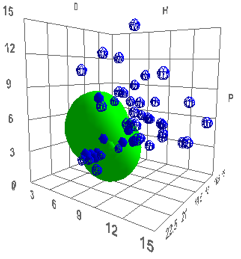
Figure 1‑4 Polystyrene and Polybutadiene spheres – the radii are made smaller
to help visualise the plot for this figure
The SBS must neither be fully compatible
nor fully non-compatible. The HSP of the bitumen must be such that the
appropriate marginal compatibility is achieved for optimum performance at both
high and low temperatures.
With an extensive table of polymer HSP it
should be easy to find polymers with a strong match of bitumen. This is easy to
do within the program. If you double-click on bitumen, the polymers in the
table are sorted with respect to their double-overlap with bitumen.
Double-overlap? Yes, you have to report two figures – how much the
bitumen sphere overlaps with the polymer sphere, and vice versa. For example,
if Polymer A is a small sphere that is completely inside Polymer B, its overlap
score is 100%. But because Polymer B’s sphere is so much larger, it is
overlapped by only a small % by Polymer A, so the score is, say, 20%. Thus the
combined overlap is shown as 120 100/20. Without this double-overlap score it
is easy to jump to the wrong conclusion about the mutual overlap of spheres.
Here is what you see on the screen.
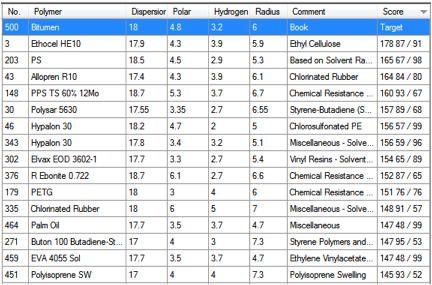
Figure 1‑5 Polymers sorted by mutual overlap with Bitumen
It is interesting to note that near the top
of the list are two styrene polymers – just as you would expect from the
previous discussion. Whether you would want to use Hypalon 30 in your bitumen,
only you can tell – but you could be confident that it would be highly
compatible.
In summary, HSP have brought light to a
very black subject. With just a few experiments you can know where to look for
interesting compatibilities or incompatibilities with polymers and have a high
degree of confidence that when you mix them you will have results along the
lines you expect. This is a far more elegant approach than experimenting in the
dark.
Another form of black material is carbon.
Like all pigments/fibres it is insoluble so the “S” in HSP certainly doesn’t
apply – or does it?
E-Book contents | HSP User's Forum


