HSP top page: Hansen Sphere with HTML5 viewer
2011.11.20
HSPiP Team Senior Developer, Dr. Hiroshi Yamamoto
The Sphere Concept was developed by Dr. Hansen. When he tried to 3D plot HSP(dD, dP, dH) of good solvents and poor solvents for one polymer with the apparatus showed below (1967), he noticed all the good solvents' HSP were very similar position and make "Sphere" in 3D space.
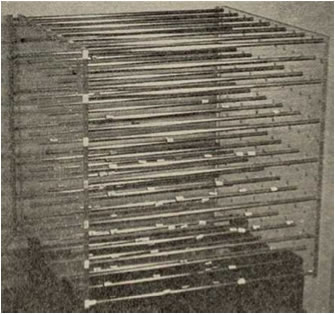
Now it past more than 40 years, thanks to HSPiP, we can see the sphere on computer. And I developed Web version of Sphere viewer. "Seeing is Believing" Please try if your browser support this programs.
If you are using adequate browser, please follow theese links.
The Sphere of Oleic Acid
Oleic Acid is easily dissolve hydorophobic solvents such as cyclohexane or xylene, but also dissolve in hydrophilic solvents such as iso-pronanol or butanol. I checked this phenomena with Double Spheres function.
Original article
The Packing polymer
This polymer seems hydrophobic polymer, though swelling very well to gasoline-ethanol solvents. Even both gasoline and ethanol are poor solvents. So this polymer may have double nature. At first, please look at single Sphere.
Original article
The Packing polymer with gasoline-Ethanol solvent
This packing rubber can use gasoline or ethanol individually. But swelling to mixture of gasoline-ethanol. You can see the reason with this sphere.
Original article
The sphere of PMMA
I determined PMMA HSP with new version of Sphere program. I got [17.7, 6.7, 6.2] and radius was 8.96. There were 4 exception solvents out of 57 solvents. HSP can predict Solvent/non-solvent 53/57=93% .
Original article
Double Spheres of PMMA
If I used Ver.3.1.x Double Spheres function,
[16.7, 9.7, 8.4] radius 7.24
[18.4, 3.2, 2.9] radius 4.22
There were 2 exceptions.
Original article
The sphere of Poly(vinylidene fluoride)
Poly(vinylidene fluoride) PVdF is used as binder resin for anode of Lithium Ion battery.I used Classical single Sphere (with option GA), I got [19.4, 15.9, 11.3] and Green Sphere radius is 9.6.
Original article
Double Spheres of Poly(vinylidene fluoride)
Poly(vinylidene fluoride) PVdF is used as binder resin for anode of Lithium Ion battery.
1st Large Green Sphere, [19.1, 15.6, 10.2] Radius 8.45
2nd Small Green Sphere, [17.5, 6.3, 9.0] Radius 4.63
The exceptions solvents reduce 7 to 2. And we can understand PVdF solubility phenomena more easily.
Original article
The sphere of Poly(N-Vinyl-2-pyrrolidone-co-crotonic Acid) Copolymer
I run classic Sphere program for Poly(N-Vinyl-2-pyrrolidone-co-crotonic Acid) Copolymer 87% - 13%. The result is [17, 15.8,19.1] Radius=11.65. There are no wrong in/out. But radius is very large. When I run the Double Spheres program,
[17, 12.2, 20] Radius 8.84 (Typical Alcohol region)
[19.4, 14.9, 5.5] Radius 5.47 (Aldehyde, Anhydride ??)
Original article
The sphere of Electrical Conducting Polymer that contain Sulfur atom
Electrical Conducting Polymer that contain Sulfur atom. I can easily understand that modified poly-Thiophene have 2 regions.One is Sphere A [17, 4.8, 6.1], the other is Sphere B [18.3, 1, 2].
Original article
The Sphere of Poly vinylchloride
hSP of Poly vinylchloride is [dD, dP, dH]=[18.8, 9.2, 63] and interaction radius (R0) is 7.3.
Original article
Double Sphere of Poly vinylchloride
The Sphere of C60
The Sphere of Chloroprene rubber
The Sphere of Endcrine Disruptor
The Sphere of epoxy resin
The Sphere of Fungicide with polymer
The Sphere of Hot taste
The Sphere of Matatabi (Cat become as if it drunk alcohol )
The Sphere of Mold release
The Sphere of mosquito dislike
The Sphere of plasticizer of polymer
The Sphere of polymer additives
The Sphere of H1N1 antiviral compound
Surface modification of TiO2
No.1
No.2
No.3
No.4
No.5
Original article


